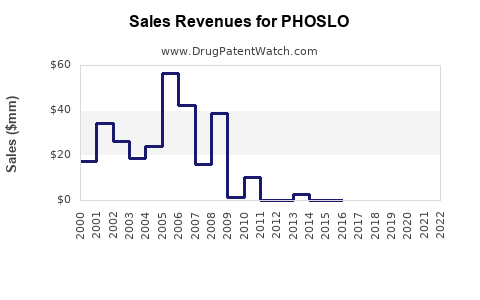
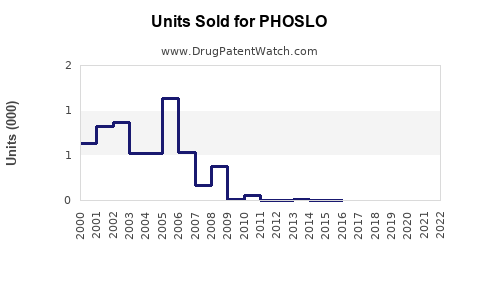
Last updated: August 1, 2025
Introduction
PHOSLO, a proprietary sodium phosphate solution, is primarily indicated for managing hyperphosphatemia associated with chronic kidney disease (CKD) and dialysis patients. Its unique formulation and targeted use have solidified its role within nephrology, especially for patients undergoing hemodialysis. This analysis explores the current market landscape, competitive positioning, growth drivers, and sales forecasts associated with PHOSLO, providing strategic insights for stakeholders.
Product Overview
PHOSLO is a phosphate-binding agent that helps reduce serum phosphorus levels, crucial in CKD management. Its prescription status and clinical utility establish it as a niche yet vital pharmaceutical within nephrology care pathways. The drug's efficacy in controlling phosphorus levels in dialysis patients gives it a sustained demand.
Market Landscape
Global CKD and Dialysis Treatment Market
The global chronic kidney disease treatment market was valued at approximately USD 49.2 billion in 2022 and is projected to expand at a CAGR of 8.2% through 2030, driven by increasing CKD prevalence and rising dialysis adoption ([1]). The dialysis products and associated pharmaceuticals like PHOSLO constitute a significant segment within this expanding market.
Prevalence and Demographic Trends
CKD affects over 700 million individuals worldwide, with end-stage renal disease (ESRD) prevalent among older adults and people with comorbidities like diabetes and hypertension ([2]). The increasing incidence, coupled with improved survival rates due to advanced therapies, sustains demand for phosphate-binding agents.
Competitive Landscape
PHOSLO faces competition primarily from other phosphate binders:
- Calcium-based binders: Calcium acetate and calcium carbonate.
- Non-calcium binders: Sevelamer (Renvela), lanthanum carbonate, and newer agents like ferric citrate.
While calcium-based agents are cost-effective, their association with vascular calcification limits their long-term use. Non-calcium binders like PHOSLO are preferred for high-risk patients, emphasizing its niche position.
Regulatory and Market Access Factors
PHOSLO's regulatory status varies by region. In the U.S., it is FDA-approved, with prescribed indications limited to hyperphosphatemia management in CKD on dialysis. Reimbursement policies influence market penetration; Medicare and insurance coverage for dialysis drugs favor established phosphate binders, sustaining sales.
Sales Drivers
- Rising CKD Prevalence: Escalates demand for phosphate management.
- Aging Population: Older patients more frequently require dialysis and phosphate control.
- Treatment Guidelines: Renal association guidelines advocate phosphate control to mitigate cardiovascular risk, underpinning continued prescription.
- Limited Competition for Niche: PHOSLO's specific formulation and proven efficacy maintain its clinical relevance.
Challenges Impacting Market Potential
- Generic Competition: The expiration of patents or market exclusivity may lead to increased generic equivalents, pressuring prices.
- Pricing Pressures: Cost containment initiatives can restrict reimbursement levels.
- Emerging Therapies: Novel phosphate binders with improved safety profiles or user convenience could threaten existing market share.
Market Penetration and Geographic Focus
North America
North America, primarily the U.S., remains the largest market due to its extensive dialysis infrastructure and high CKD prevalence. The U.S. dialysis market alone accounts for roughly USD 20 billion, with phosphate binder sales constituting a substantial fraction.
Europe
European markets benefit from advanced healthcare systems and a high CKD burden but face truncated reimbursement rates, impacting sales growth.
Asia-Pacific
This region shows rapid growth potential, driven by increasing CKD awareness, expanded healthcare access, and rising dialysis rates in China and India.
Sales Projections (2023–2028)
Based on current market dynamics, competitive landscape, and demographic trends, the following projections are outlined:
| Year |
Estimated Global Sales (USD Millions) |
Growth Rate (%) |
| 2023 |
150 |
— |
| 2024 |
165 |
10% |
| 2025 |
180 |
9% |
| 2026 |
198 |
10% |
| 2027 |
220 |
11% |
| 2028 |
242 |
10% |
Rationale: The growth trajectory reflects steady expansion in established markets, with increased penetration in emerging regions. Market maturation and potential patent expiries could temper growth beyond 2028.
Strategic Implications
- Market Expansion: Collaborating with regional distributors and aligning with regional health policies can foster market entry.
- Product Differentiation: Emphasizing PHOSLO’s proven efficacy and safety through clinical data can strengthen prescriber confidence.
- Pricing Strategies: Balancing competitive pricing with value demonstration remains essential amidst reimbursement debates.
Key Takeaways
- PHOSLO occupies a strategic niche within the CKD management spectrum, emphasizing its vital role in serum phosphate regulation.
- The expanding CKD population and dialysis treatment infrastructure support consistent demand growth.
- Competition from generic and innovative treatments necessitates strategic positioning, emphasizing efficacy, safety, and cost-effectiveness.
- Key markets, notably North America and Asia-Pacific, will drive growth; tailored regional strategies are essential.
- Moderate but steady sales growth projections highlight its ongoing relevance, with potential for increased market share through targeted initiatives.
FAQs
-
What are the main competitive advantages of PHOSLO over other phosphate binders?
PHOSLO’s proven efficacy and safety profile, coupled with its established clinical use in dialysis patients, distinguish it from newer agents that may carry uncertain long-term data.
-
How might patent expiration affect PHOSLO’s market share?
Patent expiries could lead to increased generic competition, potentially reducing prices and sales unless the brand leverages differentiation through clinical positioning or formulation improvements.
-
What regions offer the highest sales potential for PHOSLO?
North America, due to its extensive dialysis infrastructure and high CKD prevalence, and Asia-Pacific, with its rapidly growing CKD patient base, represent key growth markets.
-
Are there upcoming regulatory or clinical developments that could impact PHOSLO?
Advances in phosphate binder therapy, including novel agents with better safety profiles or administration convenience, could influence market dynamics. Regulatory changes favoring cost-effective therapies may also impact sales.
-
What strategies should stakeholders consider to maximize PHOSLO’s market potential?
Focus on expanding adoption through clinician education, establishing strong reimbursement pathways, and regional market development. Monitoring competitive innovations and patent landscapes will be essential for strategic planning.
References
[1] Grand View Research, "Chronic Kidney Disease Treatment Market Size & Trends," 2022.
[2] National Kidney Foundation, "CKD Statistics," 2023.