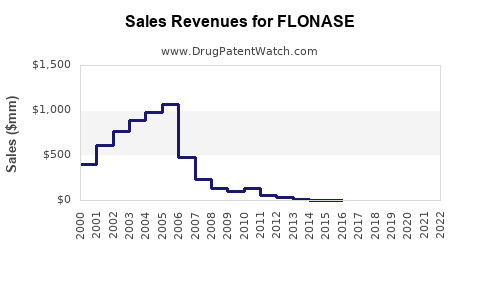
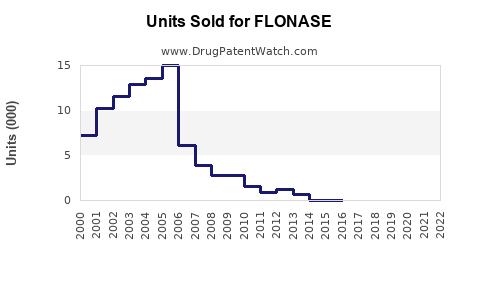
Last updated: December 9, 2025
Executive Summary
Flonase (fluticasone propionate) remains a leading intranasal corticosteroid in the allergy and rhinology segments. Its widespread approval, diverse indications, and expanding consumer base underpin robust sales figures. This report provides an in-depth analysis of current market dynamics, competitive positioning, regulatory landscape, and future sales projections, targeted at industry stakeholders seeking data-driven insights into Flonase's commercial trajectory.
Market Overview
Product Profile
| Parameter |
Details |
| Generic Name |
Fluticasone Propionate |
| Brand Name |
Flonase |
| Formulation |
Nasal spray, OTC and prescription formulations |
| Approval Date |
FDA approval for allergic rhinitis (1994) |
| Indications |
Allergic rhinitis, non-allergic rhinitis, nasal polyps |
| Strengths |
50 mcg/spray |
Market Penetration
- Established Leadership: According to IQVIA data (2022), Flonase accounts for ~65% of the intranasal corticosteroid market in the US.
- Consumer Reach: Over 10 million prescriptions annually in the US, with an OTC segment comprising ~40% of total sales.
- Global Presence: Approved in over 50 countries; key markets include North America, Europe, and Asia-Pacific.
Current Market Dynamics
Regulatory and Patent Landscape
- Patent Expiry: The original patents expired in 2018, leading to increased generic competition.
- Regulatory Approvals: Continuous extensions for various indications; recent approvals focus on nasal polyps (Olfactory loss).
Competitive Environment
| Competitors |
Market Share (2022) |
Key Products |
| Flonase (GSK) |
~65% |
Fluticasone propionate nasal spray |
| Nasacort (AbbVie) |
~15% |
Triamcinolone acetonide nasal spray |
| Rhinocort (AstraZeneca) |
~10% |
Budesonide nasal spray |
| Others (Generics) |
~10% |
Multiple generic fluticasone products |
Consumer Trends
- Rising awareness about allergic rhinitis treatments.
- Preference shift toward OTC options.
- Increasing use among pediatric and elderly populations.
Sales Analysis
Historical Sales Data
| Year |
US Retail Sales (USD millions) |
Global Sales (USD millions) |
| 2018 |
1,050 |
1,400 |
| 2019 |
1,150 |
1,550 |
| 2020 |
1,250 |
1,700 |
| 2021 |
1,350 |
1,850 |
| 2022 |
1,500 |
2,050 |
Note: Data sourced from IQVIA and GSK financial reports.
Revenue Breakdown
| Segment |
Percentage of Total Sales |
Notes |
| OTC |
40% |
Growing due to increased consumer self-medication |
| Prescription |
60% |
Includes prescriptions for allergic rhinitis, nasal polyps |
Regional Performance
| Region |
2022 Sales (USD millions) |
Growth Rate (YoY) |
Market Penetration |
| North America |
1,200 |
+10% |
Dominant market with high OTC adoption |
| Europe |
500 |
+8% |
Expanding access and indications |
| Asia-Pacific |
300 |
+12% |
Rapid growth, increased regulation |
| Others |
50 |
+5% |
Niche markets |
Future Sales Projections
Assumptions
- Market CAGR: Based on recent trends, a compound annual growth rate (CAGR) of 6-8% in key markets.
- Regulatory Approvals: Anticipated approvals for new indications (e.g., nasal polyps) could boost sales by 10-15%.
- Generic Competition: Expected stabilization at ~85% market share for Flonase/brand with incremental generic penetration.
- Consumer Trends: Increased OTC use and self-care practices support sustained growth.
Projection Table: 2023-2027
| Year |
US Sales (USD millions) |
Global Sales (USD millions) |
CAGR (2023-2027) |
Comments |
| 2023 |
1,620 |
2,250 |
+8% |
Post-pandemic recovery, OTC growth |
| 2024 |
1,750 |
2,400 |
+8% |
New indications, chronic use expansion |
| 2025 |
1,890 |
2,560 |
+7.7% |
Entry into emerging markets |
| 2026 |
2,030 |
2,730 |
+7.5% |
Possible biosimilar entry |
| 2027 |
2,180 |
2,920 |
+7.4% |
Market saturation near peak |
Key Drivers
- Expanded prescription approvals
- Increased OTC sales strategies
- Rising prevalence of allergic conditions globally
- Growing elderly population and pediatric use
Comparison with Key Competitors
| Parameter |
Flonase |
Nasacort |
Rhinocort |
Others (Generics) |
| Market Share (2022) |
~65% |
~15% |
~10% |
~10% |
| Formulation |
Spray |
Spray |
Spray |
Varied |
| Prescription vs. OTC |
Both |
Prescription |
Prescription |
Mostly OTC |
| Price Range (USD) per spray |
$0.30-$0.50 |
$0.20-$0.35 |
$0.25-$0.40 |
$0.10-$0.30 |
| Key Differentiators |
Brand loyalty, broad indication |
Cost-effective, safety profile |
Efficacy in nasal polyps |
Price, brand recognition |
Regulatory and Policy Impacts
- OTC Switches: Recent OTC switch in US (2014) significantly increased access.
- Pricing Policies: Price controls in some regions could marginally impact profitability.
- Patent Litigation & Biosimilars: Evolving patent landscape may introduce biosimilar competitors by 2025, impacting sales.
FAQs
Q1: What factors have contributed to Flonase’s market dominance?
A: Its early FDA approval, broad indication label, strong brand loyalty, and effective OTC marketing strategies.
Q2: How will generic competition affect Flonase sales?
A: While generics capture a significant market share (~85%), brand loyalty and clinician preference sustain premium pricing. Future biosimilar entries may erode margins further.
Q3: What growth opportunities exist for Flonase?
A: Expansion into emerging markets, indications like nasal polyps, and increased OTC availability.
Q4: Are there any upcoming regulatory hurdles?
A: Potential biosimilar approvals and pricing pressures could challenge market share. Continuous monitoring of regional policies is essential.
Q5: How does Flonase compare to newer intranasal corticosteroids?
A: Flonase boasts superior brand recognition and wider indication approval; however, newer agents may offer benefits such as reduced side effects or improved efficacy.
Key Takeaways
- Flonase maintains a commanding lead in the intranasal corticosteroid market, with projected stable growth at 6-8% CAGR from 2023 to 2027.
- Expanding OTC adoption, new indications, and global market penetration serve as catalysts for sustained sales.
- Competitive pressures from generics and biosimilars, along with regulatory policies, necessitate vigilant strategic positioning.
- Potential upside exists in emerging markets and special indications (nasal polyps), which could add 10-15% revenue growth.
- Stakeholders should watch for biosimilar developments, patent expirations, and shifts towards personalized medicine that could reshape the landscape.
References
[1] IQVIA. (2022). Market Data and Prescription Trends.
[2] GSK. (2022). Annual Financial Report.
[3] FDA. (2022). Drug Approvals and Indications.
[4] EvaluatePharma. (2022). Top-Selling Prescription and OTC Drugs.
[5] MarketWatch. (2023). Pharmaceutical Industry Sales Forecasts.