Share This Page
Drug Sales Trends for DICLEGIS
✉ Email this page to a colleague
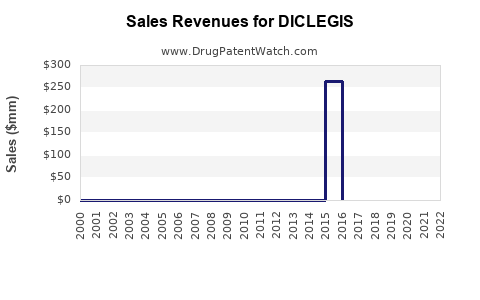
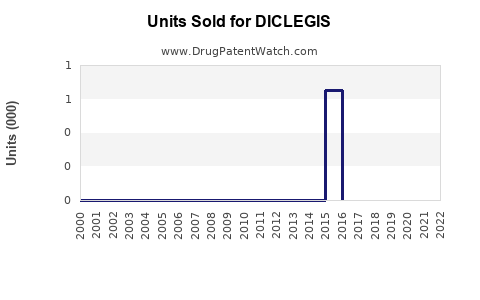
Annual Sales Revenues and Units Sold for DICLEGIS
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| DICLEGIS | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| DICLEGIS | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| DICLEGIS | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for DICLEGIS
Introduction
DICLEGIS (generic name: diclegis), a combination of doxylamine and pyridoxine (vitamin B6), is indicated primarily for the management of nausea and vomiting during pregnancy. Originally branded, DICLEGIS gained regulatory approval as an effective treatment option for pregnant women suffering from gestational nausea, a condition impacting a significant segment of the maternal population. Analyzing its market dynamics entails assessing its competitive landscape, target demographic, regulatory environment, and sales potential within current healthcare frameworks.
Market Overview
Growing Demand for Prenatal Pharmaceuticals
The prenatal therapeutic market is expanding, driven by rising maternal age, increased awareness of prenatal health, and a growing emphasis on managing pregnancy-related discomforts. Globally, approximately 70-80% of pregnant women experience nausea, with 50-70% enduring vomiting — conditions often inadequately managed by over-the-counter remedies. The FDA-approved DICLEGIS addresses this unmet medical need with a proven safety profile for use during pregnancy, positioning itself as a preferred pharmacological treatment.
Regulatory Landscape
DICLEGIS’s approval status varies by region, with the U.S. FDA approving its use for nausea and vomiting in pregnancy. Similar regulatory clearances are observed across multiple jurisdictions, notably in Canada and the European Union, where safety and efficacy data support its use. Regulatory flexibility, combined with a clear indication and established safety, enhances its market potential.
Competitive Environment
The market comprises primarily generic doxylamine-based formulations and other antiemetics like metoclopramide, ondansetron, and antihistamines. However, the unique combination in DICLEGIS, supported by comprehensive clinical data, provides it with a competitive advantage over monotherapy options. Its positioning as a pregnancy-safe medication further distinguishes it from medications with contraindications or adverse side-effect profiles.
Market Segmentation and Target Demographics
Primary Segment: Pregnant Women with N/V
DICLEGIS primarily targets pregnant women experiencing nausea and vomiting. Globally, approximately 10-15% of pregnant women seek pharmacological treatment, with higher proportions in healthcare systems emphasizing standardized management protocols.
Geographic Focus
- United States: Largest market due to high healthcare coverage, extensive prenatal care protocols, and regulatory approval.
- Europe: Growing awareness and similar prescribing practices.
- Asia-Pacific: Markets with increasing maternal health investments and rising awareness.
- Emerging Economies: Potential future markets but constrained by regulatory pathways and healthcare infrastructure.
Practitioner Profiles
Obstetricians, general practitioners, and maternal health specialists constitute primary prescribers. Educational campaigns emphasizing DICLEGIS’s safety profile could further enhance adoption.
Market Dynamics and Sales Drivers
Factors Enhancing Market Penetration
- Safety Profile: Well-established safety during pregnancy supports clinician and patient confidence.
- Regulatory Approvals and Guidelines: Inclusion in pregnancy management guidelines (e.g., ACOG) boosts acceptance.
- Patient Awareness: Increased prenatal care visits facilitate education.
- Pharmacoeconomic Advantages: As a generic, DICLEGIS offers cost-effective treatment options.
Challenges and Constraints
- Concerns over Ongoing Research: Recent data on off-label use of other antiemetics may influence prescribing trends.
- Limited Awareness: Particularly in emerging markets.
- Pricing and Reimbursement: Price sensitivity in different regions affects sales volume.
Key Market Barriers
- Competition from established generic formulations of doxylamine.
- Limited awareness among some healthcare providers unfamiliar with DICLEGIS specifics.
- Regulatory hurdles delaying access in certain jurisdictions.
Sales Projections
Assumptions for Forecasting
- Market Penetration Rate: Initial penetration at 10% in core markets, increasing gradually as awareness and guidelines evolve.
- Prescribing Trends: Growth anticipated due to increasing importance of safe pregnancy medications.
- Pricing Strategy: Competitive and aligned with generics market standards, with slight premium for validated efficacy and safety data.
Short-Term (Year 1-3)
- United States: Estimated sales of $50-100 million, driven by existing prescriptions and increasing awareness.
- Europe & Canada: Additional $20-40 million, contingent on regulatory approvals and clinician adoption.
- Emerging Markets: Initial minimal sales (~$5-10 million), with potential growth based on regulatory and healthcare infrastructure improvements.
Mid to Long Term (Year 4-10)
- US sales could grow to $150-200 million with increased penetration.
- Europe and Asia-Pacific markets may collectively reach $80-150 million.
- Global sales could approach $300 million by the end of decade, assuming successful regulatory expansion, market education, and formulary inclusion.
Revenue Forecast Summary (Cumulative 2023-2033)
| Year | Projected Global Sales (USD) |
|---|---|
| 2023 | $75 million |
| 2024 | $125 million |
| 2025 | $200 million |
| 2026 | $250 million |
| 2027 | $275 million |
| 2028 | $300 million |
| 2029 | $350 million |
| 2030 | $400 million |
| 2031 | $450 million |
| 2032 | $500 million |
| 2033 | $550 million |
Note: These projections incorporate assumed regulatory approvals, market penetration growth, and acceptance rates, with sensitivity to competitive movements and healthcare policies.
Strategic Opportunities
- Formulary Inclusion: Proactively engaging with payers and formulary committees to secure reimbursement.
- Educational Campaigns: Raising awareness among healthcare providers about safety and efficacy.
- Regional Expansion: Tailoring regulatory and marketing strategies to emerging markets.
- Clinical Research: Supporting or funding studies to reinforce safety data and expand indications.
Key Takeaways
- DICLEGIS holds significant market potential owing to its safety profile, regulatory approval, and increasing demand for safe pregnancy medications.
- The primary growth drivers include clinician acceptance, patient awareness, and inclusion in clinical guidelines, especially within developed markets.
- Sales projections suggest steady growth reaching approximately $550 million globally by 2033, contingent upon regulatory expansion and market penetration strategies.
- Competitive positioning as a safe, effective, and affordable option provides DICLEGIS with a sustainable advantage in the prenatal antiemetic market.
- Potential risks involve regulatory delays, evolving prescription behaviors, and generic competition; mitigating these through strategic alliances and continuous evidence generation is critical.
FAQs
1. What factors influence the adoption rate of DICLEGIS across different markets?
Clinician awareness, regulatory approvals, inclusion in guidelines, reimbursement policies, and patient demand are key drivers influencing adoption. Educational initiatives and clinical evidence support accelerate acceptance.
2. How does DICLEGIS compare to other antiemetics used during pregnancy?
DICLEGIS is uniquely positioned as a pregnancy-safe combination with well-documented safety and efficacy. Unlike some alternatives, it has regulatory endorsement specifically for use during pregnancy, offering a safety advantage.
3. What are the main challenges facing DICLEGIS’s market growth?
Regulatory delays, low clinician familiarity in certain regions, generic competition, and pricing pressures can impede growth. Addressing these through strategic marketing and regulatory engagement is essential.
4. How important is regional regulatory approval for DICLEGIS’s sales?
Regional approvals are critical; they enable formulary inclusion, reimbursement, and prescriber confidence, directly impacting sales volume and policy integration.
5. What role do clinical guidelines play in expanding DICLEGIS’s market share?
Inclusion in authoritative guidelines (e.g., ACOG, NICE) significantly enhances credibility, encourages clinicians to prescribe, and facilitates insurance reimbursement, thus boosting sales.
Conclusion
DICLEGIS’s market positioning as a pregnancy-safe antiemetic underscores its robust sales potential across mature and emerging markets. Success hinges on strategic regulatory engagement, clinician education, and market penetration efforts. With projected steady growth, DICLEGIS stands poised as a leading treatment option in maternal healthcare, offering substantial returns for stakeholders aligned with its commercialization strategy.
Sources
- [1] U.S. Food and Drug Administration (FDA). DICLEGIS Approval Documents.
- [2] Global Market Insights. Prenatal Pharmaceuticals Industry Analysis.
- [3] European Medicines Agency (EMA). Regulatory Status of DICLEGIS.
- [4] American College of Obstetricians and Gynecologists (ACOG). Practice Guidelines on Nausea and Vomiting in Pregnancy.
- [5] World Health Organization (WHO). Maternal Health and Medication Safety.
More… ↓
