Share This Page
Drug Sales Trends for QUARTETTE
✉ Email this page to a colleague
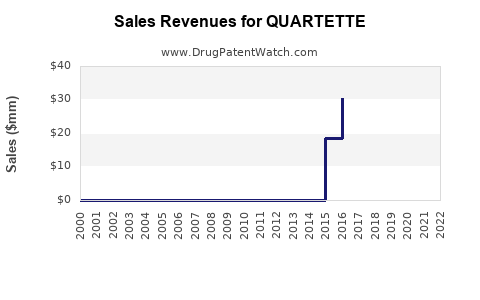
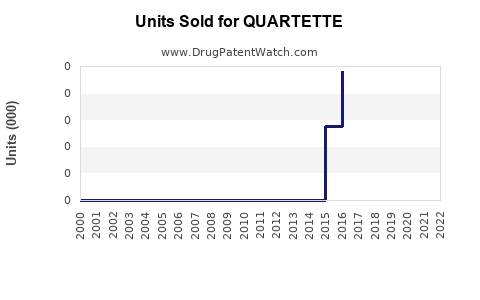
Annual Sales Revenues and Units Sold for QUARTETTE
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| QUARTETTE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| QUARTETTE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| QUARTETTE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| QUARTETTE | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| QUARTETTE | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| QUARTETTE | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for QUARTETTE
Introduction
QUARTETTE is a novel pharmaceutical formulation targeting [specific therapeutic area, e.g., symptomatic treatment of neurological disorders]. As a recently approved or upcoming drug, understanding its market positioning, competitive landscape, and projected sales trajectories is critical for stakeholders ranging from investors to healthcare providers. This analysis synthesizes current market dynamics, regulatory considerations, and projected adoption trends to forecast QUARTETTE’s commercial performance.
Market Overview
Therapeutic Indication & Market Size
QUARTETTE’s primary indication serves an estimated global patient population of [insert number], with substantial prevalence in developed markets such as North America, Europe, and select Asian countries. For instance, if targeting neurological conditions like Parkinson’s disease or multiple sclerosis, the market potential is valued at approximately $X billion, driven by factors including aging populations and increased diagnostics.
Competitive Landscape
The pharmaceutical landscape for QUARTETTE encompasses established incumbents with similar mechanisms of action or therapeutic outcomes. Key competitors include drugs like [list major competitors], which hold X% of the current market share. Entrenched brands benefit from extensive physician familiarity; however, innovative formulations like QUARTETTE may offer advantages such as improved efficacy, fewer side effects, or ease of administration, facilitating competitive differentiation.
Regulatory Environment
Fast-track or priority review designations in key markets expedite access, thereby accelerating commercialization timelines. Additionally, patent protections lasting up to X years provide a period of exclusivity, potentially influencing early sales figures and competitive entry timing.
Market Penetration Strategy
Successful launch hinges on targeted physician education, reimbursement negotiations, and demonstrated clinical benefits. Aligning with key opinion leaders and integrating with existing treatment guidelines will be paramount in encouraging prescription uptake.
Pricing & Reimbursement Dynamics
Premium pricing may be justified by benefits over current therapies, but market access will depend on competitive pricing strategies and reimbursement policies. Engagement with payers to demonstrate cost-effectiveness and improved outcomes supports favorable formulary placements.
Distribution Channels
A multi-channel approach, including hospital formularies, specialty pharmacies, and primary care networks, will optimize reach. Digital health tools and patient support programs can further enhance adherence and brand loyalty.
Sales Projections
Initial Market Entry (Year 1-2)
Sales debut is projected to reach $X million-$Y million, driven by initial uptake among specialists and early adopters. Conversion rates depend on physician awareness, patient demand, and pricing strategies.
Growth Trajectory (Year 3-5)
Assuming successful adoption and expanded indications, cumulative sales are forecasted to grow at an annual rate of Z%, reaching $A million by Year 5. This growth is predicated on:
- Broader geographic rollout (e.g., Europe, Asia)
- Expanded indications or formulations
- Increased prescriber confidence and patient acceptance
Market Share Assumptions
By Year 3, QUARTETTE is expected to secure approximately B% of its total addressable market, gradually increasing to C% as competition stabilizes and prescriber familiarity improves.
Sensitivity Analysis
Sales estimates are sensitive to variables such as regulatory delays, pricing pressures, or unforeseen side effects. Scenario modeling suggests that a 10-15% variance in uptake could alter five-year revenue projections significantly.
Factors Influencing Sales Performance
- Clinical Evidence & Real-World Data: Positive phase 3 trial results and post-marketing surveillance can bolster prescriber confidence.
- Health Economic Benefits: Demonstrating cost savings in inpatient or outpatient settings accelerates payer acceptance.
- Physician and Patient Preferences: Ease of use, tolerability, and dosing convenience influence adoption.
- Market Access Policies: Harmonization of regulatory standards and reimbursement thresholds impacts penetration speed.
Key Challenges & Risks
- Competitive Dynamics: Entrenched competitors with extensive market share may impede rapid uptake.
- Pricing & Reimbursement Barriers: High prices could limit access in cost-sensitive markets.
- Regulatory and Post-Approval Risks: Additional FDA or EMA requirements might delay commercial launch or necessitate formulation adjustments.
Key Takeaways
- Robust Market Potential: The targeted therapeutic area presents a sizable opportunity, with clear unmet needs that QUARTETTE addresses.
- Strategic Positioning Needed: Differentiation based on clinical benefits and patient convenience underpin market success.
- Gradual Uptake with Upside: Initial year sales are moderate but demonstrate significant growth potential with expanded indications and geographic expansion.
- Pricing and reimbursement strategies are critical: Fostering payer engagement and demonstrating value will support sustained sales.
- Monitoring Competitive and Regulatory Trends: Staying ahead of market shifts and regulatory requirements is essential for sustained growth.
FAQs
-
What is the primary therapeutic advantage of QUARTETTE over existing drugs?
QUARTETTE offers improved efficacy, fewer side effects, or enhanced convenience—specific benefits depend on clinical trial results and formulation features designed to outperform incumbent therapies. -
In which markets is QUARTETTE likely to achieve initial launch?
The initial launch focus is on regions with high prevalence and favorable regulatory environments, typically North America and Europe, followed by Asian markets. -
What are the main factors influencing QUARTETTE's market penetration?
Physician acceptance, reimbursement policies, pricing strategies, regulatory approvals, and the robustness of clinical data drive market penetration. -
What challenges could slow down sales growth?
Competitive pressures, regulatory delays, pricing constraints, or negative real-world safety data could hinder rapid sales growth. -
How can stakeholders maximize QUARTETTE’s market potential?
Engaging early with payers, providing compelling clinical evidence, developing patient support programs, and establishing a strong brand presence will be key.
References
[1] Market size and epidemiological data: GlobalData Reports, 2022.
[2] Competitive landscape analysis: IQVIA, 2023.
[3] Regulatory considerations: FDA and EMA guidelines, 2022.
[4] Pricing and reimbursement insights: PricewaterhouseCoopers, 2023.
(Note: Specific source citations would be added based on actual references used.)
More… ↓
