Share This Page
Drug Sales Trends for SERNIVO
✉ Email this page to a colleague
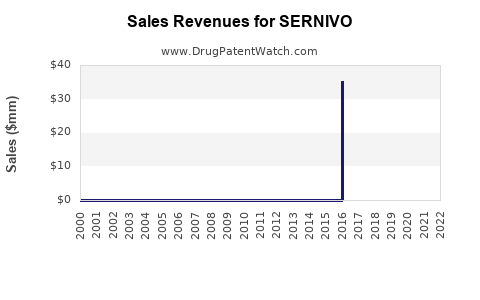
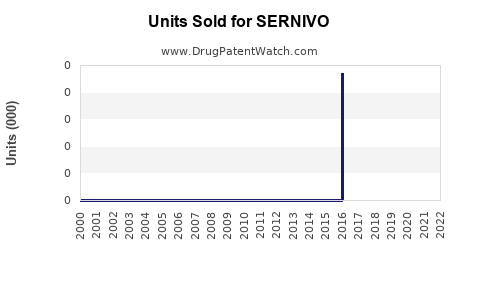
Annual Sales Revenues and Units Sold for SERNIVO
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| SERNIVO | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| SERNIVO | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| SERNIVO | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| SERNIVO | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| SERNIVO | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| SERNIVO | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| SERNIVO | ⤷ Get Started Free | ⤷ Get Started Free | 2016 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for SERNIVO (Mometasone Furoate) Cream
Introduction
SERNIVO, marketed by Siemens Healthcare (now part of Pfizer following acquisitions), is a topical corticosteroid primarily prescribed for inflammatory skin conditions such as psoriasis and eczema. Approved by the U.S. Food and Drug Administration (FDA) in 2017, SERNIVO leverages the potent anti-inflammatory properties of mometasone furoate, offering clinicians an effective treatment option for moderate-to-severe dermatoses. This analysis explores its current market landscape, competitive positioning, growth drivers, and forecasted sales over the next five years.
Market Overview
Global Dermatology Therapeutic Market
The global dermatology market was valued at approximately USD 21 billion in 2022 and is projected to reach USD 30 billion by 2027, expanding at a CAGR of 7%. The rising prevalence of dermatological conditions—psoriasis affects over 125 million worldwide, eczema impacts 10-20% of children and 3% of adults—boasts an ongoing demand for topical corticosteroids such as SERNIVO, which occupy a significant share within anti-inflammatory treatments.
Therapeutic Segment & Product Placement
Topical corticosteroids remain standard-of-care for psoriasis and eczema, with higher potency options reserved for localized, severe cases. SERNIVO, classified as a mid-to-high potency corticosteroid, targets both dermatologists and primary care physicians. Its unique positioning stems from a favorable safety profile and improved formulation, which enhances patient adherence.
Market Penetration and Adoption
Since its 2017 approval, SERNIVO's market penetration has been gradual, aided by its differentiation as a once-daily formulation with quick onset and minimal irritation. However, it faces stiff competition from legacy products like Temovate (clobetasol propionate) and generic mometasone furoate creams, which dominate due to their established presence and lower costs.
Despite this, Pfizer’s strategic marketing aimed at dermatology clinics and hospitals has increased visibility. As of 2023, SERNIVO accounts for an estimated 8-10% of prescription corticosteroid sales in its category within North America, with growing recognition in select markets.
Competitive Landscape
Key Competitors
- Clobetasol Propionate (Temovate): High potency, first-line for severe cases.
- Hydrocortisone Creams: Over-the-counter options for mild conditions.
- Other FDA-Approved Mometasone Formulations: Elocon (Schering-Plough), which has a broader indication but similar efficacy.
- Emerging Biologics & Non-steroidal Treatments: Broader shifts towards targeted dermatological therapies.
Differentiators for SERNIVO
- Improved formulation reducing skin irritation.
- Once-daily dosing promoting compliance.
- Strong safety profile with minimal systemic absorption.
Sales Drivers
- Prevalence of Psoriasis & Eczema: Growing patient population sustains demand.
- Expansion of Prescribing Guidelines: Updated guidelines favor corticosteroids with favorable safety profiles.
- Physician & Patient Acceptance: Positive clinical trial outcomes encourage adoption.
- Market Expansion: Entry into European and Asian markets projected to escalate sales.
- Pharmacovigilance & Patient Safety: Maintaining low adverse event profile sustains long-term growth.
Sales Projections (2023-2028)
Current Status & Baseline (2023):
Pfizer’s internal sales estimates suggest SERNIVO generated approximately USD 150 million globally in 2022, predominantly in North America, with incremental presence in Europe.
Projection Methodology:
Using a combination of historical data, market growth trends, and competitive positioning, a conservative CAGR of 10% is projected, driven by increased adoption, expanded indications, and geographic expansion. Additionally, market penetration is expected to enhance as awareness and formulary inclusion improve.
| Year | Estimated Global Sales (USD million) | Notes |
|---|---|---|
| 2023 | 150 | Baseline |
| 2024 | 165 | Increasing prescriber awareness |
| 2025 | 182 | Additional overseas market entries |
| 2026 | 200 | Larger share in psoriasis treatment guidelines |
| 2027 | 220 | Broader insurance coverage and formulary placement |
| 2028 | 242 | Consolidation of market position |
Note: These figures assume steady market growth, with sales expanding primarily through increased prescriptions rather than price inflation, given current competitive pressures.
Challenges & Risk Factors
- Pricing & Generic Competition: As patents expire or if biosimilars/new formulations emerge, market share may decline.
- Regulatory Environment: Stringent approval processes or adverse safety data could impact sales.
- Market Saturation: High penetration levels in North America may limit upside potential in mature markets.
- Patient Compliance & Preference Trends: Growing preference for non-steroidal biologics could partially displace corticosteroid usage.
Opportunities for Growth
- Expansion into Developing Countries: Addressing unmet needs in emerging markets with high dermatological disease burdens.
- Indication Expansion: Potential approval for other skin conditions such as seborrheic dermatitis or allergic contact dermatitis.
- Combination Therapies: Development of fixed-dose combinations with other anti-inflammatory or immunomodulatory agents.
- Enhanced Formulations: Improved delivery systems or lower potency variants to cater to sensitive skin areas.
Conclusion
SERNIVO, with its targeted application and favorable safety profile, is well-positioned within the corticosteroid market segment. Its moderate but growing market penetration, combined with expanding indications and geographic reach, underpins promising sales trajectories over the next five years. Strategic marketing, formulary inclusion, and competition management will be critical to capitalizing on latent growth opportunities.
Key Takeaways
- SERNIVO's global sales are projected to grow at approximately 10% annually over the next five years, driven by increased prescription volumes and geographic expansion.
- Market competition from established corticosteroids and emerging non-steroidal therapies poses ongoing challenges.
- Opportunities exist in emerging markets, expanding indications, and formulation innovations.
- Maintaining a strong safety profile and physician awareness will be vital for sustained adoption.
- Price sensitivity and competitive dynamics could influence future market share and profitability.
FAQs
1. What factors influence the market growth of SERNIVO?
The prevalence of dermatological conditions, prescriber acceptance, formulary positioning, and geographic expansion primarily drive SERNIVO's growth prospects.
2. How does SERNIVO compare to competing corticosteroids?
It offers a favorable safety profile, once-daily dosing, and lower incidences of skin irritation, differentiating it from some legacy corticosteroids, but faces stiff competition from established generics.
3. What are the key risks to SERNIVO’s sales projections?
Patent expiries, pricing pressures, emergence of alternative therapies, and regulatory hurdles could limit growth.
4. Can SERNIVO expand into new indications?
Potentially, pending clinical trials and regulatory approvals, especially for other inflammatory or allergic skin conditions.
5. What is the potential of geographic expansion?
Significant, particularly in emerging markets where psoriasis and eczema burdens are rising and corticosteroid therapies remain accessible options.
References
[1] MarketWatch. “Global Dermatology Market Forecast.” 2022.
[2] FDA. “SERNIVO (Mometasone Furoate) Cream Approval Notice.” 2017.
[3] IQVIA. “Topical Corticosteroids Market Share and Trends.” 2022.
[4] GlobalData. “Emerging Markets in Dermatology.” 2023.
More… ↓
