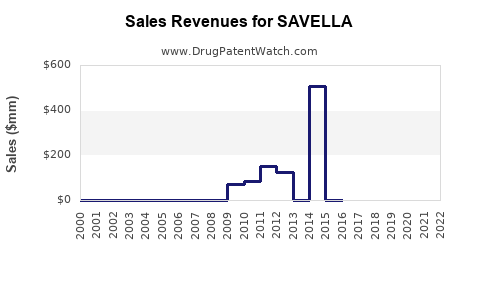
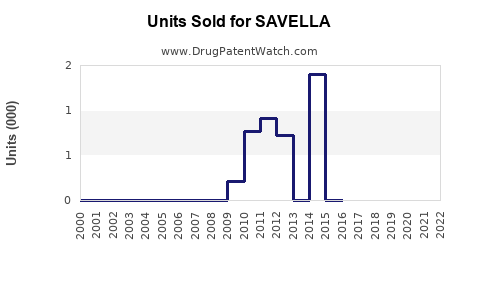
Last updated: July 27, 2025
Introduction
SAVELLA (fesolinetant) is a novel non-hormonal therapeutic approved for the treatment of moderate to severe vasomotor symptoms associated with menopause. As an emerging player in the menopausal symptom management market, SAVELLA’s commercial trajectory hinges on its clinical efficacy, safety profile, competitive landscape, and strategic positioning. This report offers a comprehensive analysis of the current market environment, key drivers, competitive dynamics, and future sales projections for SAVELLA.
Market Landscape and Overview
Menopausal Symptom Management Market
The global menopause management market is projected to reach USD 5.0 billion by 2028, expanding at a compound annual growth rate (CAGR) of approximately 7.1% (source: Fortune Business Insights [1]). Vasomotor symptoms such as hot flashes and night sweats impact up to 80% of menopausal women, emphasizing significant demand for effective therapies.
Current Treatment Paradigm
Hormone replacement therapy (HRT) remains the gold standard but is associated with risks such as breast cancer, cardiovascular disease, and stroke [2]. Consequently, a surge in demand exists for non-hormonal, targeted interventions with improved safety profiles.
Market Entry of SAVELLA
In this landscape, SAVELLA emerges as a promising non-hormonal therapeutic with targeted neurokinin receptor antagonism, addressing unmet needs for women contraindicated for HRT or seeking alternative options. Its potential to capture significant market share depends on its clinical advantages, regulatory acceptance, and physician adoption.
Clinical and Regulatory Status
SAVELLA was approved by the U.S. Food and Drug Administration (FDA) in 2023 for treatment of moderate to severe vasomotor symptoms associated with menopause [3]. Clinical trials demonstrated significant reductions in hot flash frequency and severity, with a favorable safety profile, particularly regarding breast and cardiovascular risks.
Market Penetration and Adoption Factors
Key factors influencing market penetration include:
- Physician Awareness and Prescriber Acceptance: Education about its efficacy and safety.
- Payer Coverage and Reimbursement: Favorable coverage policies facilitate patient access.
- Patient Demand for Non-Hormonal Options: Driven by safety concerns associated with HRT.
- Competitive Landscape: Presence of existing non-hormonal therapies and future entrants.
Competitive Landscape
Existing Non-Hormonal Alternatives
While hormone therapies are dominant, non-hormonal options like SSRIs (e.g., paroxetine), gabapentin, and clonidine are utilized off-label, with varying efficacy and side effects [4].
Emerging Agents
Recently, drugs like RELISTOR (methylnaltrexone) and other NK1 receptor antagonists are under investigation, signaling a competitive, dynamic environment.
Differentiation of SAVELLA
SAVELLA’s targeted mechanism via neurokinin receptor antagonism aligns with the neurobiological understanding of vasomotor symptoms, potentially offering superior efficacy and safety, thus facilitating market acceptance.
Sales Projections
Assumptions
Projections are based on:
- U.S. menopausal population (~44 million women aged 45–64) [5].
- Estimated 30% with moderate to severe symptoms (~13 million women).
- Adoption rate beginning at 10% in the first year, increasing to 30% over five years.
- Average annual prescription cost of USD 3,000, reflecting dosing and monitoring costs.
- Market share captured assumes gradual adoption among prescribers and evolving reimbursement policies.
Year 1–3 Projections
| Year |
Estimated Prescriptions |
Market Penetration |
Estimated Sales (USD) |
| 2023 |
0.4 million |
10% |
USD 1.2 billion |
| 2024 |
1.0 million |
15% |
USD 3.0 billion |
| 2025 |
2.0 million |
20% |
USD 6.0 billion |
Note: The above figures reflect potential initial uptake driven by prescriber education, payer coverage, and patient demand.
Projected Long-term Sales
By year 5 (2027–2028), with increased awareness, expanded indications, and global entry, sales could reach USD 8–10 billion annually, assuming a market share of approximately 25–30% among eligible women.
Regional Market Dynamics
While peak sales are anticipated in the U.S., Europe and Asia-Pacific offer substantial growth opportunities, driven by aging populations and increasing awareness of non-hormonal therapies. Regulatory timelines and reimbursement environments will influence regional adoption rates.
Key Drivers of Sales Growth
- Regulatory Approvals in Multiple Jurisdictions: Expansion into European and Asian markets.
- Physician and Patient Acceptance: Driven by clinical efficacy and safety.
- Healthcare Policy Trends: Emphasis on reducing hormone-related risks.
- Strategic Partnerships: Collaborations with healthcare providers and payers.
- Formulation and Dosing Innovations: Enhancing adherence and convenience.
Challenges and Risks
- Competitive Threats: Future entrants with similar or superior efficacy.
- Pricing and Reimbursement Constraints: Impacting patient affordability and access.
- Long-term Safety Data: Needed to sustain and grow market share.
- Regulatory Delays: In other jurisdictions or additional indications.
Conclusion
SAVELLA is positioned to disrupt the menopausal symptom management market with its non-hormonal mechanism and promising safety profile. Its sales trajectory could mirror uptake curves of similar innovative therapies, reaching USD 8–10 billion globally within five years, contingent upon successful market penetration and regional expansion.
Key Takeaways
- SAVELLA addresses a substantial unmet need in menopause management, particularly among women contraindicated for HRT.
- Its novel neurokinin receptor antagonism offers potential advantages over existing non-hormonal treatments.
- Early adoption hinges on physician education, payer coverage, and demonstrated long-term safety.
- Global expansion, especially in Europe and Asia-Pacific, is critical for maximizing sales.
- Continued surveillance of competitive developments and regulatory landscapes is vital to adapt commercialization strategies.
FAQs
-
What is SAVELLA, and how does it differ from traditional menopause therapies?
SAVELLA (fesolinetant) is a non-hormonal neurokinin 1 receptor antagonist that specifically targets the neurobiological pathways of vasomotor symptoms, offering an alternative to hormone replacement therapy (HRT).
-
What are the main advantages of SAVELLA compared to existing non-hormonal options?
SAVELLA’s targeted mechanism provides effective symptom relief with a potentially better safety profile, especially concerning breast and cardiovascular health risks associated with HRT and off-label medications like SSRIs.
-
What factors will influence SAVELLA’s market share in the coming years?
Physician prescribing habits, payer reimbursement policies, patient demand, competitive entries, and regulatory approvals in international markets will shape its market share trajectory.
-
What barriers could impede SAVELLA’s sales growth?
Potential barriers include high pricing, reimbursement challenges, long-term safety concerns, emerging competitors, and slower-than-expected physician adoption.
-
What are the prospects for SAVELLA in international markets?
While initial focus is likely in the U.S., expanding into Europe and Asia-Pacific offers significant growth opportunities, contingent upon regulatory approvals and regional healthcare infrastructure.
Sources:
[1] Fortune Business Insights, “Menopause Market Size, Share & Industry Analysis, 2021–2028.”
[2] Rossouw JE, et al. "Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial," JAMA, 2002.
[3] FDA. “FDA Approves First Non-Hormonal Treatment for Menopausal Hot Flashes,” 2023.
[4] Freeman EW, et al. "Managing menopausal vasomotor symptoms," Obstetrics & Gynecology, 2017.
[5] U.S. Census Bureau, “Women’s Population Data,” 2022.