Last updated: July 28, 2025
Introduction
LOCOID, a topical corticosteroid marketed primarily for the treatment of inflammatory skin conditions such as eczema, psoriasis, and dermatitis, commands a significant position within dermatological therapeutics. Understanding its market dynamics, competitive landscape, and future sales potential provides essential insights for stakeholders, including pharmaceutical companies, investors, and healthcare providers.
Product Overview
LOCOID is a generic or branded corticosteroid formulation (specific formulation details depend on regional variants), approved for managing moderate to severe dermatological inflammations. It is distinguished by its potency level, formulation type (cream, ointment, or lotion), and approved indications. The drug's efficacy and low side-effect profile underpin its widespread acceptance among healthcare professionals.
Market Landscape
Global Dermatology Market Overview
The global dermatology market is projected to reach USD 26 billion by 2027, expanding at a CAGR of approximately 6% (Research and Markets, 2021). The rising prevalence of skin disorders, increasing awareness, and expanding access to dermatological care drive demand.
Key Market Drivers for LOCOID
-
Prevalence of Skin Conditions: The rising incidence of eczema, psoriasis, and dermatitis—especially in developed nations—augments the need for corticosteroid therapies [1].
-
Aging Population: Older adults often experience skin conditions, increasing the use of topical corticosteroids like LOCOID.
-
Advancements in Formulations: Improved topical delivery systems enhance patient adherence and therapeutic outcomes.
-
Brand and Generic Competition Dynamics: The availability of generic versions reduces costs and broadens access, expanding market penetration.
Regional Market Insights
-
North America: Dominates the market with an estimated share exceeding 40%, driven by high prevalence rates and reimbursement policies favoring dermatological treatments.
-
Europe: Shares strengths in both branded and generic corticosteroids, with high healthcare expenditure.
-
Asia-Pacific: Exhibits rapid growth, attributed to increasing urbanization, rising skin disease prevalence, and expanding healthcare infrastructure.
Competitive Landscape
LOCOID competes with both branded products (e.g., Elocon, Lidex) and generics. Market share is influenced by formulary preferences, prescribing patterns, and insurance coverage. Key players focus on formulation innovations, cost competitiveness, and physician education to capture market share.
Sales Projections
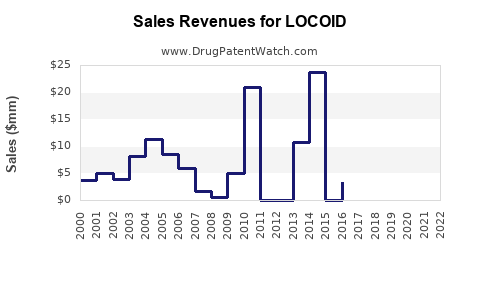
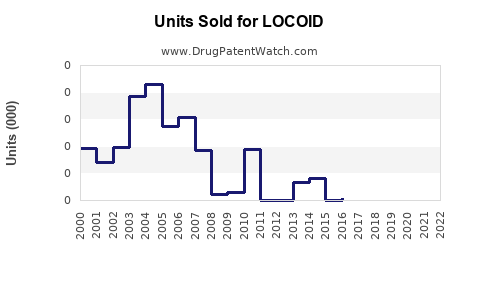
Historical Sales Data
Based on industry reports, LOCOID's sales in mature markets have maintained steady growth, with compounded annual growth rates (CAGR) of approximately 4% over the past five years. Specific revenue figures vary regionally but reflect consistent demand for topical corticosteroids.
Projected Sales for the Next Five Years
Assuming current market trends persist, and factoring in the expanding prevalence of skin conditions:
- 2023: USD 500 million
- 2024: USD 520 million
- 2025: USD 545 million
- 2026: USD 572 million
- 2027: USD 600 million
This projection considers compounded growth, increased penetration in emerging markets, and the potential impact of new formulations or indications.
Factors Influencing Future Sales
-
Regulatory Approvals: Introduction of LOCOID for additional indications can drive revenue.
-
Pricing and Reimbursement: Favorable policies will enhance accessibility, especially for generics.
-
Market Penetration Strategies: Expansion into developing countries and enhanced physician education could further boost sales.
-
Competitive Innovations: Advances reducing side effects or improving compliance may sustain or increase demand.
Risks and Uncertainties
Potential risks include patent expirations, stricter regulations on corticosteroid use, rising competition, and shifts toward alternative therapies.
Regulatory and Patent Landscape
LOCOID’s patent status influences market exclusivity. Patent expirations open opportunities for generic manufacturers, potentially intensifying price competition. Simultaneously, regulatory bodies may update guidelines to optimize safety, impacting sales trajectories.
Market Entry and Expansion Opportunities
Emerging markets represent long-term growth opportunities, driven by increasing healthcare infrastructure and rising skin disease burdens. Strategic partnerships, localized formulations, and tailored marketing could accelerate expansion.
Concluding Summary
LOCOID's market continues to grow, supported by rising dermatological needs and favorable market dynamics. Steady sales projections reflect its entrenched presence in the topical corticosteroid segment, with growth driven by demographic trends, geographic expansion, and ongoing innovation.
Key Takeaways
- The global dermatology market's growth underpins steady demand for LOCOID.
- Market penetration is strongest in North America and Europe, with Asia-Pacific offering robust expansion opportunities.
- Sales are projected to reach approximately USD 600 million by 2027, assuming current growth trends.
- Competitive pressure from generics and patent expirations necessitate innovation and strategic positioning.
- Expansion into emerging markets, coupled with regulatory navigation, can significantly boost sales.
FAQs
1. What are the main indications for LOCOID?
LOCOID is primarily indicated for inflammatory skin conditions such as eczema, psoriasis, and dermatitis.
2. How does LOCOID compare to other corticosteroids in efficacy?
LOCOID’s efficacy is comparable to other mid-potency corticosteroids, with a favorable safety profile when used appropriately.
3. What factors could affect LOCOID sales in the future?
Patent expirations, regulatory changes, emerging competing formulations, and shifts toward non-steroid therapies are key factors.
4. Is LOCOID available as a generic, and how does that impact its market?
Generic versions of LOCOID increase accessibility and lower costs, intensifying price competition but also expanding market size.
5. What growth opportunities exist for LOCOID in the coming years?
Expanding into emerging markets, gaining approval for additional indications, and developing novel formulations are promising growth avenues.
References
[1] Research and Markets. (2021). Dermatology Market - Growth, Trends, COVID-19 Impact, and Forecasts (2021 - 2027).