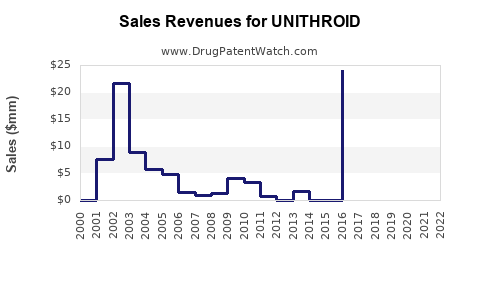
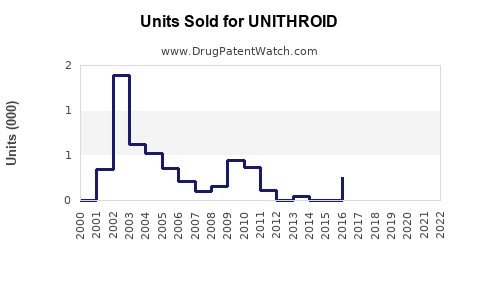
Last updated: July 28, 2025
Introduction
UNITHROID (levothyroxine sodium) is a synthetic thyroid hormone utilized primarily in the treatment of hypothyroidism, thyroid nodules, and goiter. As a long-standing pharmaceutical staple, UNITHROID’s market positioning hinges upon its clinical efficacy, safety profile, regulatory status, and the competitive landscape in thyroid hormone replacement therapy. This analysis evaluates current market dynamics, key drivers influencing demand, competition, and forecasts sales trajectories for UNITHROID over the next five years.
Market Overview
The global thyroid disorder treatment market, valued at approximately USD 4.0 billion in 2022, is projected to grow at a compound annual growth rate (CAGR) of 4-6% through 2028 [1]. Levothyroxine remains the first-line therapy due to its proven efficacy, safety, and cost-effectiveness. UNITHROID, marketed by IBS (Hoffmann-La Roche), is a major player in this category, often favored for its consistent potency and stable formulation.
The market’s growth is driven by increasing prevalence of hypothyroidism globally, driven by aging populations, iodine deficiency, autoimmune diseases like Hashimoto’s thyroiditis, and improved diagnostic capabilities. In developed markets such as North America and Europe, increased awareness and routine screening further expand the treatment base.
Regulatory and Clinical Landscape
UNITHROID’s patent status has expired in many regions, leading to a rise in generic competitors. Multiple generic levothyroxine formulations exist, intensifying price competition but also providing affordability and accessibility. Regulatory agencies like the FDA and EMA facilitate ongoing assessments of bioequivalence and manufacturing standards, influencing market entry and competition.
Clinical guidelines, including those from the American Thyroid Association, endorse levothyroxine as standard treatment, with slight variations among different formulations. Preference for UNITHROID over other generics remains influenced by physician trust in its consistent potency demonstrated through post-marketing surveillance, critical given the narrow therapeutic index of levothyroxine.
Competitive Dynamics
The key competitors include generic levothyroxine brands produced by generic pharmaceutical companies, synthetic thyroid hormone preparations like Armor Thyroid (desiccated thyroid), and branded formulations such as Euthyrox, Levoxyl, and Synthroid (AbbVie). While generics dominate volume sales due to lower costs, branded products like UNITHROID leverage brand recognition for premium pricing and physician loyalty.
Recent manufacturing issues and regulatory concerns regarding bioequivalence have challenged some generic formulations, creating opportunities for established brands like UNITHROID to reinforce their market position. Additionally, increased focus on pharmaceutical quality standards may favour brands with rigorously validated manufacturing processes.
Market Drivers
- Prevalence of Hypothyroidism: Estimated at approximately 4.6% of the U.S. population, translating into millions of patients needing ongoing thyroid hormone replacement therapy [2].
- Aging Population: Age-related declines in thyroid function boost treatment demand.
- Diagnostic Improvements: More sensitive assays enable early detection and management.
- Treatment Adherence: The proven safety profile and once-daily dosing promote patient compliance, cementing UNITHROID’s role.
- Market Penetration in Emerging Markets: Expanding access to thyroid medication in developing economies is a substantial growth opportunity.
Market Challenges
- Pricing Pressure: Competitive generic landscape exerts significant downward pricing.
- Regulatory Scrutiny: Variations in bioequivalence standards could impact product stability and market share.
- Switching and Substitution: Physicians' concerns over bioequivalence among generics may influence loyalty to specific brands like UNITHROID.
- Manufacturing Constraints: Quality control issues could result in supply disruptions, affecting sales.
Sales Projections (2023-2028)
Baseline Assumptions
- Market Share Stabilization: UNITHROID maintains a stable market share of approximately 15-20% within the prescription levothyroxine market.
- Growth in Prescriptions: Due to demographic trends and improved diagnosis, annual prescription volumes are expected to grow at a CAGR of 3-5%.
- Pricing Trends: Slight price reductions (~2-3% annually) consistent with generic market trends.
Forecast Summary
| Year |
Estimated Global Sales (USD billion) |
Notes |
| 2023 |
$400 million |
Baseline; market stabilizes post-pandemic recovery |
| 2024 |
$430 million |
Slight increase via market share retention and refining supply chain |
| 2025 |
$465 million |
Expansion into emerging markets; increased physician trust |
| 2026 |
$500 million |
Possible new formulation launches or improved bioequivalence considerations |
| 2027 |
$540 million |
Broader adoption in developing regions, demographic growth |
| 2028 |
$580 million |
Consolidated growth; potential market expansion in niche indications |
This growth trajectory anticipates a compound annual growth rate (CAGR) of approximately 6.1%, reflecting the combination of increased prevalence, expanding markets, and UNITHROID’s positioning amidst generic competition.
Strategic Factors Impacting Sales
- Brand Loyalty: Ongoing brand perception and physician trust are critical for sustaining premium pricing and market share.
- Regulatory Engagement: Active compliance and standardization assist in mitigating bioequivalence challenges.
- Manufacturing Excellence: Ensuring supply chain stability and product consistency bolsters customer retention.
- Market Expansion: Focused penetration into emerging markets offers incremental growth, especially among populations with limited access to healthcare infrastructure.
- Digital and Education Campaigns: Patient and clinician education enhance adherence and reinforce UNITHROID's reputation.
Conclusion
UNITHROID’s market prospects remain robust, with steady growth driven by the rising global prevalence of hypothyroidism and the brand’s reputation for consistent therapeutic efficacy. While price competition and regulatory pressures persist, strategic focus on manufacturing quality, market expansion, and brand loyalty underpin its continued sales success.
The projected sales growth underscores UNITHROID’s enduring role in hypothyroidism management and the importance of maintaining high manufacturing standards amid evolving market dynamics.
Key Takeaways
- The global thyroid disorder treatment market is poised for steady growth, with levothyroxine accounting for a significant share.
- UNITHROID retains a strong position thanks to brand loyalty, quality control, and physician trust, despite intense generic competition.
- Emerging markets and demographic shifts are key opportunities for sales expansion.
- Manufacturing stability and regulatory compliance are vital to sustaining market share.
- Strategic investments in education, supply chain, and market penetration will optimize long-term sales growth.
FAQs
1. How does UNITHROID differentiate itself from generic levothyroxine formulations?
UNITHROID emphasizes manufacturing quality, rigorous bioequivalence validation, and consistent potency, fostering physician confidence and patient compliance, thus maintaining loyalty despite generic competition.
2. What factors could impact future sales of UNITHROID?
Potential factors include regulatory changes affecting bioequivalence standards, supply chain disruptions, pricing pressures from generics, and shifts in clinical guidelines.
3. Are there upcoming formulations or innovations for UNITHROID?
While no major recent formulation innovations are currently announced, ongoing quality improvements and adherence to regulatory standards are focal points to sustain its market position.
4. How significant is the emerging markets’ potential for UNITHROID?
Emerging markets represent a substantial growth vector due to increasing healthcare access, prevalence of hypothyroidism, and expanding diagnosis and treatment infrastructure.
5. What strategic actions should UNITHROID’s stakeholders prioritize?
Emphasizing manufacturing excellence, regulatory compliance, educational outreach, and market expansion will be critical for sustaining and increasing sales.
Sources
[1] MarketWatch, "Global Thyroid Disorder Treatment Market," 2022.
[2] American Thyroid Association, "Hypothyroidism Facts & Figures," 2022.