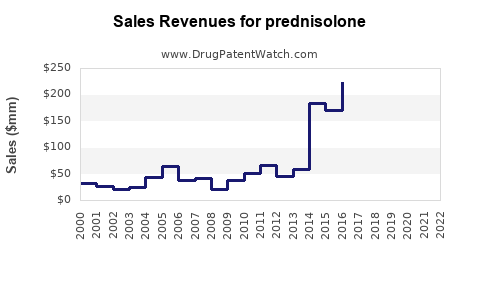
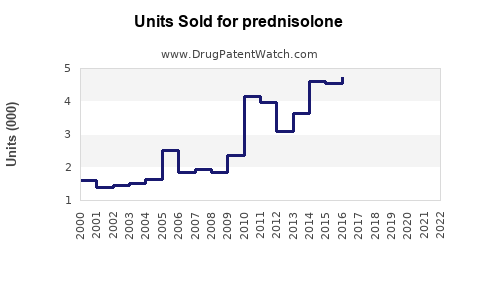
Last updated: July 29, 2025
Introduction
Prednisolone, a synthetic glucocorticoid with potent anti-inflammatory and immunosuppressive properties, holds a significant position in the global pharmaceutical landscape. It is widely used for managing conditions such as allergies, asthma, autoimmune diseases, and inflammatory disorders. The drug’s broad therapeutic application, coupled with its long-established clinical efficacy, makes it a key product in both developed and emerging markets. This comprehensive analysis explores the current market landscape, competitive positioning, future demand drivers, and sales projections for Prednisolone.
Market Landscape and Current Position
Global Market Size and Revenue
The global corticosteroids market, which encompasses Prednisolone, was valued at approximately USD 1.8 billion in 2022 (source: MarketsandMarkets). Prednisolone accounts for a significant share due to its widespread off-patent availability and cost-effectiveness. As a generic medication, its sales are predominantly driven by off-patent formulations, with branded versions holding niche markets.
Forecasts estimate compounded annual growth rates (CAGR) of around 3-4% over the next five years, driven chiefly by rising incidences of autoimmune and respiratory diseases globally.
Key Markets
-
North America: North America remains the largest market, driven by high healthcare expenditure, extensive healthcare infrastructure, and high disease prevalence. The U.S. accounts for a significant proportion of sales, with compounded growth fueled by extensive prescription practices.
-
Europe: Europe's mature healthcare systems lead to consistent demand. Europe’s corticosteroid market growth is steady, with a focus on both branded and generic formulations.
-
Asia-Pacific: This region exhibits the highest growth potential due to increasing healthcare access, rising chronic disease burden, and economic development. Countries like China and India are experiencing double-digit growth rates, primarily in generic corticosteroids.
-
Latin America and Middle East & Africa: These regions are emerging markets, with growth driven by expanding healthcare infrastructure and an increasing focus on affordable therapeutics.
Competitive Landscape
Prednisolone's generic status garners intense competition among pharmaceutical giants like Mylan, Sandoz, Teva, and local manufacturers. Market dominance in emerging markets often correlates with manufacturing capacity, distribution reach, and pricing strategies.
Innovative formulations, such as ocular or injectable Prednisolone, occupy niche segments with limited competition. Patent expiry timelines in key markets have facilitated a broadening of product availability and price erosion.
Market Drivers
-
Increasing Prevalence of Autoimmune Disorders and Allergic Conditions: Rising autoimmune diseases such as rheumatoid arthritis, lupus, and multiple sclerosis boost demand for immunosuppressive agents like Prednisolone.
-
Growing Respiratory Diseases: Asthma and COPD patients increasingly rely on corticosteroid inhalers and oral formulations, bolstering sales.
-
Cost-Effective Treatment Options: Prednisolone’s generic status offers affordable treatment alternatives, especially in developing economies.
-
Expanding Healthcare Access: Improving healthcare infrastructure in emerging markets broadens the patient base.
Market Challenges
-
Side Effects and Long-term Toxicity Risks: Adverse effects such as osteoporosis, hyperglycemia, and immunosuppression may limit prolonged use, influencing prescribing patterns.
-
Regulatory Pressures: Stringent safety evaluations and quality standards could increase manufacturing costs.
-
Competition from Newer Agents: Biologics and targeted therapies for autoimmune conditions are gradually replacing corticosteroids in some indications.
Future Demand and Sales Projections
Assumptions for Projection
- Steady growth in autoimmune and respiratory disease prevalence.
- Continued patent expirations favoring generic proliferation.
- Moderate penetration of innovative formulations.
- Stable regulatory environment and healthcare expenditure.
Projection Summary (2023–2028)
| Year |
Estimated Market Size (USD billion) |
Prednisolone Share |
Prednisolone Revenue (USD million) |
CAGR Estimate |
| 2023 |
1.9 |
~85% |
1,615 |
— |
| 2024 |
2.0 |
~85% |
1,700 |
5% |
| 2025 |
2.1 |
~85% |
1,785 |
5% |
| 2026 |
2.2 |
~84% |
1,850 |
4.5% |
| 2027 |
2.3 |
~84% |
1,935 |
4.5% |
| 2028 |
2.4 |
~84% |
2,020 |
4.5% |
These projections suggest Prednisolone’s sales will grow modestly over the next five years, primarily driven by volume increases in emerging markets. Price erosion due to generic competition is balanced by an expanding patient population.
Market Entry and Expansion Opportunities
-
Regional Expansion: Companies focusing on Asian and Latin American markets can leverage the growing demand for affordable corticosteroids.
-
Product Diversification: Developing combination therapies (e.g., Prednisolone with antihistamines) and novel delivery forms can capture niche markets.
-
Regulatory Approvals: Navigating regulatory pathways efficiently, especially in emerging markets, will promote market penetration.
-
Brand Differentiation: While generics dominate, differentiation through quality assurance, biosimilar development, or derivative formulations can enhance competitive positioning.
Key Regulatory Trends
Regulatory agencies such as the FDA, EMA, and PMDA are emphasizing safety profiles and biosimilar regulations. Companies must ensure compliance to sustain market access, especially as monographs evolve around corticosteroid safety standards (source: FDA).
Impact of Market Dynamics
The corticosteroid market's growth hinges on global healthcare expenditure, chronic disease management strategies, and the availability of cost-effective generics. The ongoing global health challenges, such as COVID-19, which exacerbates respiratory conditions, can indirectly influence demand.
Key Takeaways
-
Stable Revenue Stream: Prednisolone benefits from its established efficacy, low cost, and broad therapeutic applications, ensuring steady demand and sales continuity.
-
Growth in Emerging Markets: Increased healthcare access and disease prevalence in developing regions provide significant sales expansion opportunities.
-
Competitive Pressures: The generic landscape and pricing dynamics necessitate strategic focus on cost efficiencies and regional market penetration.
-
Regulatory Navigations: Staying ahead of evolving safety regulations and biosimilar policies is key to maintaining market share.
-
Innovations and Formulations: Developing new delivery routes and combination therapies could offset generic price erosion and expand market reach.
FAQs
1. What factors influence the sales growth of Prednisolone?
Sales are primarily driven by disease prevalence (autoimmune, respiratory), regional healthcare infrastructure, generic competition, regulatory policies, and the expansion into emerging markets.
2. How will patent expiries affect the Prednisolone market?
Patent expiries have facilitated the surge of generic manufacturers, increasing supply and reducing prices, but also intensify competition, impacting profit margins.
3. Are there emerging therapeutic applications for Prednisolone?
While its primary use remains anti-inflammatory and immunosuppressive, ongoing research into new indications and combination therapies may create additional demand in niche markets.
4. What is the impact of safety concerns on Prednisolone’s sales?
Adverse effect profiles may limit long-term usage, prompting prescribers to consider alternatives, which could restrain growth but also open opportunities for safer formulations.
5. How does regional healthcare expenditure influence Prednisolone sales?
Higher healthcare spending enhances patient access and prescription rates, especially in developed markets, while expanding coverage in emerging economies fosters volume growth.
Conclusion
Prednisolone’s long-standing presence in the global pharmaceutical market ensures consistent demand, with substantial growth prospects rooted in expanding healthcare access and increasing disease burden—particularly within emerging economies. Strategic manufacturing, regional expansion, and innovation in formulations represent vital avenues for sustaining and enhancing sales trajectories amidst a competitive landscape.
References
[1] MarketsandMarkets, “Corticosteroids Market,” 2022.
[2] Global Data, “Autoimmune Disease Prevalence and Treatment Market,” 2023.
[3] FDA Guidelines, “Safety and Efficacy Standards for Corticosteroids,” 2022.