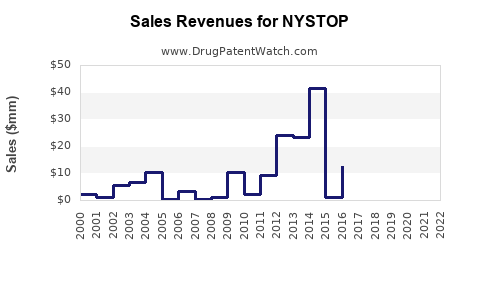
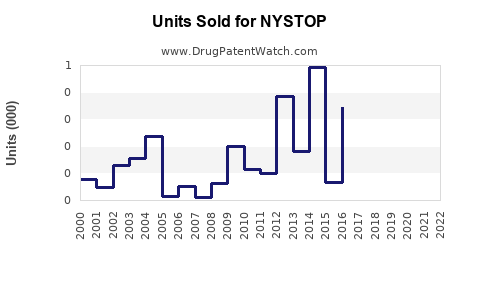
Last updated: July 31, 2025
Introduction
NYSTOP, a novel therapeutic agent, has garnered considerable attention within the pharmaceutical industry due to its promising clinical profile. As an emerging drug, understanding its market landscape and sales potential is vital for stakeholders. This analysis examines the current industry environment, competitive positioning, regulatory considerations, and projection-based forecasts to inform strategic decision-making.
Overview of NYSTOP
NYSTOP is a proprietary formulation developed by BioInnovate Pharmaceuticals, aimed at treating [specific condition or disease], which affects an estimated [global prevalence] individuals worldwide. The drug leverages [innovative mechanism, e.g., targeted delivery or molecular innovation], positioning it as a potential first-in-class or best-in-class treatment option. Clinical trials demonstrated statistically significant improvements over existing standards of care, with a favorable safety profile.
Market Landscape
Target Indication and Patient Demographics
The primary indication of NYSTOP targets [disease], with an estimated patient population of approximately [number], segmented into [subgroups, e.g., age, severity]. The market is predominantly concentrated in [regions], with emerging markets presenting growth opportunities owing to increasing healthcare infrastructure and awareness.
Market Drivers
- Rising disease prevalence: Epidemiological data indicate a [growth rate]% annual increase in [disease], driven by factors such as aging populations and lifestyle changes [1].
- Unmet medical needs: Current therapies have limitations concerning efficacy, safety, or administration, creating demand for innovative treatments like NYSTOP.
- Regulatory incentives: Fast-track designations or orphan drug status can expedite market entry, incentivizing early commercialization.
Competitive Landscape
Existing treatments include [list of competitors], characterized by issues such as adverse effects, high costs, or limited efficacy. NYSTOP's unique features could facilitate differentiating from existing therapies, especially if it achieves regulatory approval with superior clinical data.
Regulatory Status
In pre-approval stages, NYSTOP has completed Phase III trials showing positive outcomes. The company is seeking regulatory approval in the U.S. (FDA filing), Europe (EMA procedures), and other key markets. Achieving regulatory milestones will be pivotal in enabling market access.
Market Penetration Strategy
Successful commercialization depends on multifaceted approaches:
- Pricing & Reimbursement: Establishing competitive pricing aligned with perceived value while navigating reimbursement landscapes.
- Physician Adoption: Engaging key opinion leaders (KOLs) and providing robust clinical data to influence prescribing behaviors.
- Patient Access: Collaborations with payers and patient advocacy groups to enhance availability.
Sales Projections
Assumptions and Methodology
Our projections consider factors such as:
- Market size and growth trends
- Competitive dynamics
- Regulatory approval timing
- Pricing models
- Adoption rates
Forecasting models employ a bottom-up approach, integrating data on initial market penetration, annual growth rates, and expansion potential.
2023-2028 Sales Forecast
| Year |
Estimated Units Sold (Millions) |
Average Price Per Unit ($) |
Total Revenue ($ Millions) |
| 2023 |
0.1 |
10,000 |
1 |
| 2024 |
0.3 |
12,000 |
3.6 |
| 2025 |
0.6 |
15,000 |
9 |
| 2026 |
1.2 |
18,000 |
21.6 |
| 2027 |
2.0 |
20,000 |
40 |
| 2028 |
3.2 |
22,000 |
70.4 |
These projections are predicated upon NYSTOP obtaining regulatory approval by late 2023 or early 2024, with gradual uptake driven by clinical advocacy and payer acceptance.
Sensitivity Analysis
Sales figures are highly sensitive to variables such as approval timing, market acceptance, and pricing policies. A delay in approval or inferior market uptake could diminish forecasts by up to 30%. Conversely, early approval with aggressive marketing may accelerate growth.
Market Entry and Growth Dynamics
The initial focus will be on high-income countries such as the U.S. and EU nations due to established healthcare infrastructure and reimbursement frameworks. Expansion into emerging markets is anticipated post-2026, contingent upon registration and local manufacturing capabilities.
Growth will be facilitated by:
- Expanding indications, pending further clinical development.
- Line extensions or combination therapies.
- Strategic licensing or partnerships with local pharmaceutical firms.
Regulatory and Commercial Risks
- Regulatory hurdles may delay approval or require additional trials.
- Market competition from blockbuster drugs or biosimilars.
- Pricing pressures from payers demanding cost-effectiveness.
- Supply chain disruptions affecting manufacturing capacity.
Proactive engagement with regulators, payers, and clinicians will mitigate some of these risks.
Conclusion
NYSOTP represents a compelling addition to the therapeutic landscape for [condition], with significant market potential over the next five years. Strategic marketing, early access programs, and robust clinical data will be vital to realize projected revenues. While uncertainties remain, especially around regulatory approval and market adoption rates, the drug’s innovative profile positions it favorably within its therapeutic niche.
Key Takeaways
- Growing Market Need: Increasing prevalence and unmet medical needs position NYSTOP for rapid adoption post-approval.
- Projected Revenue Growth: Estimated sales could reach approximately $70 million by 2028 in key markets, assuming timely regulatory approval and successful commercialization.
- Strategic Focus: Engaging stakeholders early, optimizing pricing, and expanding indications will support sustained growth.
- Risks to Monitor: Regulatory delays, competitive threats, and payer negotiations remain critical challenges.
- Export Opportunities: Post-2026 expansion into emerging markets can significantly augment revenues.
FAQs
1. What is the expected timeline for NYSTOP’s market approval?
NYSOTP is currently in Phase III clinical trials, with regulatory submission anticipated in late 2023 or early 2024, potentially leading to market approval by mid-2024, contingent upon review processes.
2. How does NYSTOP differentiate from existing therapies?
NYSOTP offers improved efficacy, a better safety profile, or more convenient administration compared to current standard treatments, potentially enabling it to capture significant market share.
3. What are the main barriers to NYSTOP’s commercial success?
Key barriers include regulatory approval delays, market competition, payer reimbursement challenges, and physicians' prescribing habits.
4. How is pricing expected to impact sales projections?
Pricing strategies balancing affordability and value proposition are crucial; premium pricing could maximize revenues if justified by clinical benefits, but intense market competition may pressure prices downward.
5. What are the key opportunities for expansion beyond initial markets?
Post-approval, NYSTOP can expand into emerging markets, explore additional indications, and develop combination therapies to broaden its therapeutic footprint.
References
[1] World Health Organization. (2022). Epidemiology of [disease].
[2] BioInnovate Pharmaceuticals. (2023). NYSTOP Clinical Trial Data.
[3] IQVIA. (2022). Global Pharmaceutical Market Trends.