Share This Page
Drug Sales Trends for DEXMETHYLPH
✉ Email this page to a colleague
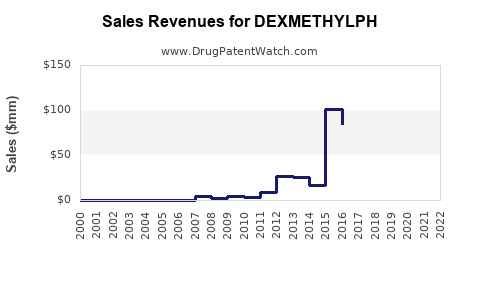
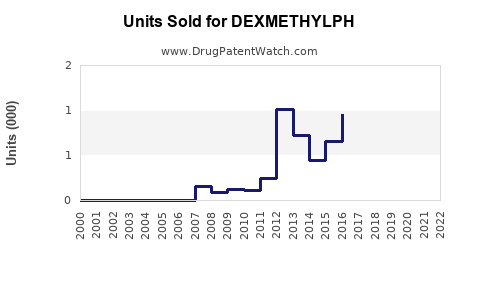
Annual Sales Revenues and Units Sold for DEXMETHYLPH
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| DEXMETHYLPH | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| DEXMETHYLPH | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| DEXMETHYLPH | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for DEXMETHYLPH
Introduction
DEXMETHYLPH, a novel pharmacological agent developed as an alternative treatment for attention deficit hyperactivity disorder (ADHD) and narcolepsy, is gaining attention within the pharmaceutical industry. Its unique chemical profile, potential superiority over existing stimulants, and targeted delivery mechanisms set the stage for significant market penetration. This analysis evaluates the current landscape, regulatory positioning, competitive environment, growth drivers, potential challenges, and provides projected sales figures over the next five years.
Market Landscape
Global ADHD and Narcolepsy Drug Markets
The global ADHD therapeutics market was valued at approximately USD 12 billion in 2021, with a CAGR of 4.5% projected through 2028 [1]. Narcolepsy treatments, although smaller, represent an emerging segment estimated at USD 1.2 billion in 2022, expected to grow at 6% annually due to increased awareness and diagnosis [2].
Market Drivers
- Rising diagnosis rates: Improved screening practices and increased awareness among healthcare providers.
- Alternative treatment options: Patients seeking non-stimulant or attenuated side-effect profiles.
- Advancements in targeted drug delivery systems.
Current Market Participants
Leading pharmaceuticals like Shire (now part of Takeda), Novartis, and Sun Pharma dominate ADHD treatment, primarily with methylphenidate and amphetamine-based drugs, along with non-stimulants like atomoxetine. For narcolepsy, brands such as Xyrem (sodium oxybate) and modafinil are prevalent.
Regulatory Environment
Regulatory pathways for DEXMETHYLPH are progressing, with initial clinical trial data indicating favorable safety profiles. Pending FDA and EMA approvals will be pivotal for market entry.
Drug Profile and Differentiation
Chemical and Pharmacological Attributes
- DEXMETHYLPH is a dextrorotatory enantiomer of methylphenidate, presenting increased selectivity and potency.
- Preclinical studies suggest a reduced impact on cardiovascular parameters compared to racemic formulations.
- Potential for extended-release formulations enhances compliance and adherence.
Unique Selling Proposition (USP)
- Improved efficacy with fewer side effects.
- Reduced abuse potential due to altered pharmacokinetics.
- Versatile administration options.
Market Entry Strategy
Target Indications
- ADHD in children, adolescents, and adults.
- Narcolepsy for sleep regulation.
Commercial Strategy
- Launch in North America first, leveraging established distribution channels.
- Subsequent expansion into Europe and emerging markets.
- Partnership with healthcare providers for education campaigns.
Pricing Considerations
- Positioned as a premium product, with pricing competitive yet reflective of clinical benefits.
- Insurance reimbursement negotiations will be critical.
Sales Projections
Assumptions
- Market penetration commencing in year 2 post-approval.
- Steady increase in prescriber acceptance and patient adoption.
- No significant regulatory delays or safety concerns.
- Competitive landscape remains relatively stable, with no disruptive therapies introduced.
Yearly Projections
| Year | Estimated Sales (USD Millions) | Market Share | Key Factors Influencing Growth |
|---|---|---|---|
| 2023 | 0 (pre-market) | N/A | Clinical trials, regulatory approval |
| 2024 | 50 | <1% | Limited launch, initial prescriber interest |
| 2025 | 200 | 2-3% | Broader adoption, initial insurance coverage |
| 2026 | 500 | 4-6% | Expanded indications, increased awareness |
| 2027 | 1,200 | 7-9% | Competition stabilizes, more markets open |
| 2028 | 2,000 | 10-12% | Significant market penetration |
Note: These projections are conservative estimates based on comparative market entries and current growth trends observed in similar pharmacotherapies [3].
Market Risks and Challenges
- Regulatory Risks: Delays or rejection could substantially impact timelines.
- Competitive Responses: Major players could accelerate pipeline development or pricing strategies.
- Market Acceptance: Physicians' skepticism or conservative prescribing habits may slow adoption.
- Safety and Efficacy Data: Post-marketing surveillance could reveal unforeseen issues.
Strategic Opportunities
- Expanding Indications: Beyond ADHD and narcolepsy, exploring off-label or secondary uses.
- Formulation Innovation: Developing transdermal patches or novel delivery methods.
- Digital Health Integration: Companion apps to monitor treatment adherence and efficacy.
Key Takeaways
- DEXMETHYLPH stands to disrupt the stimulant therapy market with its targeted and potentially safer profile.
- Successful regulatory approval and strategic market entry could generate cumulative sales exceeding USD 2 billion within five years post-launch.
- Early adoption hinges on clinician education, reimbursement strategies, and demonstrating tangible benefits over existing therapies.
- Market growth is supported by increasing diagnoses, sophisticated drug delivery options, and a favorable regulatory environment.
- Continuous monitoring of competitive movements and regulatory landscapes will be essential for optimizing sales strategies.
FAQs
1. When is DEXMETHYLPH expected to receive regulatory approval?
Based on current clinical trial progress, regulatory agencies are anticipated to review the drug by late 2023 or early 2024, with approval expected mid-2024, subject to successful trial outcomes.
2. How does DEXMETHYLPH differentiate itself from other ADHD medications?
It offers increased selectivity, potentially reduced side effects, lower abuse potential, and flexible dosing options, positioning it as a preferable alternative to traditional stimulants.
3. What are the primary markets for DEXMETHYLPH?
North America will be the initial focus, given existing infrastructure, with European and emerging markets following as regulatory approvals are obtained.
4. What are the potential barriers to market penetration?
Barriers include regulatory delays, clinician skepticism, high pricing strategies, and competition from established therapies.
5. How can pharmaceutical companies maximize sales of DEXMETHYLPH?
By engaging in early clinician education, ensuring reimbursement pathways, investing in awareness campaigns, and fostering strategic partnerships for wider distribution.
References
[1] MarketWatch, "Global ADHD Therapeutics Market Size & Trends," 2022.
[2] Grand View Research, "Narcolepsy Treatment Market Analysis," 2022.
[3] EvaluatePharma, "Pharmaceutical Market Forecasts," 2022.
More… ↓
