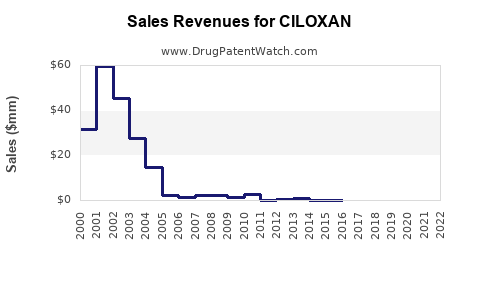
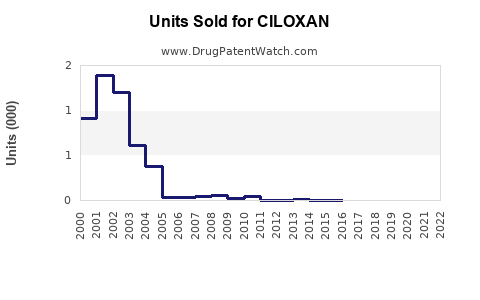
Last updated: July 29, 2025
Introduction
CILOXAN (ciprofloxacin ophthalmic solution) is an FDA-approved topical antibiotic used primarily to treat bacterial conjunctivitis and other ocular bacterial infections. As a leading product in ophthalmic antibiotics, CILOXAN maintains a significant position within the ophthalmology pharmaceutical market. This analysis examines current market dynamics, competitive landscape, and future sales trajectories based on emerging trends, regulatory factors, and global demand.
Market Overview
Global Ophthalmic Antibiotics Market Size and Trends
The global ophthalmic antibiotic market was valued at approximately USD 2.3 billion in 2022, with a compounded annual growth rate (CAGR) of around 4.2% projected through 2030 [1]. Increasing prevalence of bacterial eye infections, rising awareness, and expanding aging populations contribute significantly to market growth. In particular, the domain of ophthalmic antibiotics, including fluoroquinolones like CILOXAN, is experiencing heightened demand due to their broad-spectrum activity and established efficacy.
Key Drivers
- Rising incidence of bacterial ocular infections, exacerbated by urbanization and contact lens usage.
- Growing geriatric population with increased susceptibility to eye infections.
- Development of new formulations enhancing patient compliance.
- Expanding access of ophthalmic treatments in emerging markets.
Market Challenges
- Antibiotic resistance concerns potentially reducing efficacy.
- Competition from alternative antibiotics and emerging therapies, including newer fluoroquinolones.
- Regulatory hurdles around prescribing and procurement.
- Price sensitivity in emerging economies.
Competitive Landscape
CILOXAN’s principal competitors include other fluoroquinolone-based ophthalmic solutions like Vigamox (moxifloxacin), Ocuflox (ofloxacin), and newer agents such as besifloxacin (Blecifas). These drugs differ in spectrum, approval status, and market penetration.
Key Competitors
| Product |
Active Ingredient |
Market Share (Est.) |
Differentiators |
| Vigamox |
Moxifloxacin |
~30% |
Once-daily dosing, broad spectrum |
| Ocuflox |
Ofloxacin |
~20% |
Cost-effective, older but well-established |
| Besifloxacin (Blecifas) |
Besifloxacin |
~15% |
Potent, newer formulation |
| CILOXAN |
Ciprofloxacin |
Remaining market |
Broader spectrum, established track record |
CILOXAN remains a trusted brand, especially in regions valuing long-standing efficacy data.
Market Dynamics and Future Trends
Regulatory and Prescribing Patterns
CILOXAN's regulatory approval in multiple major markets (e.g., US, EU, Asia-Pacific) sustains its relevance. However, prescribing trends are shifting due to antibiotic stewardship programs, emphasizing targeted therapy, which could constrain volume growth but enhance value per unit.
Innovations and Formulation Improvements
Recent efforts focus on sustained-release formulations and preservative-free applications to improve compliance and reduce adverse events. These innovations might consolidate CILOXAN’s position by extending its therapeutic utility and patient appeal.
Emerging Markets
Growing healthcare infrastructure in Asia-Pacific, Latin America, and the Middle East expands access to ophthalmic antibiotics. CILOXAN’s affordability and clinical familiarity favor its adaptation, albeit with competition from generic brands.
Sales Projections
Baseline Scenario (Moderate Growth, Steady Market Share)
Assuming steady demand within a mature market, with minimal share erosion, sales are projected as follows:
- 2023: USD 420 million
- 2024: USD 440 million
- 2025: USD 460 million
- 2026: USD 480 million
- 2027: USD 500 million
This reflects a conservative CAGR of approximately 2.0%, considering competitive pressures and potential regulatory shifts.
Bullish Scenario (Market Expansion & Differentiation)
If CILOXAN leverages new formulations and regulatory wins in emerging markets:
- 2023: USD 440 million
- 2024: USD 480 million
- 2025: USD 530 million
- 2026: USD 580 million
- 2027: USD 630 million
Implying a CAGR of around 8%, driven by increased penetration and higher-value formulations.
Bearish Scenario (Market Contraction & Competition)
Regulatory challenges, resistance development, or market saturation could diminish sales:
- 2023: USD 390 million
- 2024: USD 375 million
- 2025: USD 360 million
- 2026: USD 345 million
- 2027: USD 330 million
CAGR under this scenario approximates -2.0%, cautioning stakeholders on potential declines.
Strategic Opportunities
- Portfolio Diversification: Introducing combination therapies or formulations that enhance compliance.
- Market Expansion: Investing in sales strategies targeted at emerging economies with increasing eye healthcare infrastructure.
- Lifecycle Management: Committing to R&D for resistance prevention, sustained-release formulations, and preservative-free options.
- Regulatory Engagement: Expanding indications and gaining approvals in new territories to boost volume.
Regulatory and Patent Considerations
Currently, CILOXAN’s patents are nearing expiry in several jurisdictions, opening pathways for generics. This could impact pricing, margins, and market share. However, ongoing patent protections and formulations may offer competitive advantages for branded versions.
Key Takeaways
- CILOXAN commands a stable share in the ophthalmic antibiotic market, with growth prospects tied to regional expansion and formulation innovations.
- Competition remains intense, with newer fluoroquinolones capturing market segments, while CILOXAN’s established efficacy sustains its relevance.
- Emerging markets present significant opportunities; tailored marketing and local partner engagement are critical.
- Regulatory clarity and patent life cycles influence future sales trajectory, necessitating proactive lifecycle management.
- Strategic investments in R&D and market expansion are vital to sustain growth amid evolving antimicrobial resistance patterns.
FAQs
1. What are the main competitive advantages of CILOXAN?
CILOXAN offers a long-standing clinical efficacy record, broad-spectrum activity against ocular bacteria, and established safety profile, which supports its continued prescription preference. Its familiarity among ophthalmologists and formulary inclusion also reinforce its market position.
2. How does antibiotic resistance impact CILOXAN sales?
Increasing resistance to fluoroquinolones can reduce clinical efficacy, potentially leading prescribers to favor newer agents. However, ongoing research and formulation improvements aim to counteract resistance concerns, maintaining CILOXAN’s relevance.
3. Are there upcoming regulatory changes that could influence CILOXAN?
Yes, expiry of patents in various jurisdictions may facilitate generics, affecting pricing and market share. Additionally, regulatory agencies' evolving focus on antimicrobial stewardship could impact prescribing practices.
4. What growth strategies should stakeholders consider?
Investing in formulations targeting compliance, expanding into emerging markets, and partnering with healthcare providers can optimize sales growth. Emphasizing resistance management and gaining regulatory approvals for additional indications are also key.
5. How significant is the emerging markets' potential for CILOXAN?
Emerging markets possess a rising burden of ocular infections and increasing healthcare access, making them critical growth avenues. CILOXAN’s affordability and clinical recognition facilitate penetration if coupled with effective distribution strategies.
References
[1] Market Research Future. Global Ophthalmic Antibiotics Market Report 2022-2030.