Share This Page
Drug Sales Trends for NAPHCON-A
✉ Email this page to a colleague
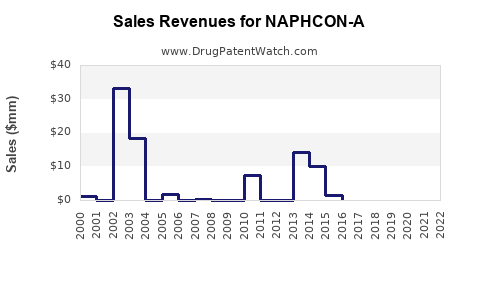
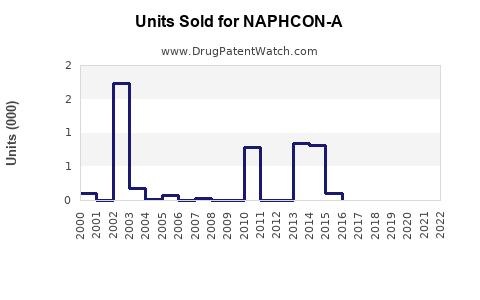
Annual Sales Revenues and Units Sold for NAPHCON-A
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| NAPHCON-A | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| NAPHCON-A | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| NAPHCON-A | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| NAPHCON-A | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| NAPHCON-A | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| NAPHCON-A | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for NAPHCON-A
Introduction
NAPHCON-A is a prescription ophthalmic solution combining naphazoline hydrochloride and pheniramine maleate, primarily used for symptomatic relief of allergic conjunctivitis and ocular irritations. As a combination antihistamine-decongestant, NAPHCON-A penetrates the global allergy and ophthalmology markets, offering targeted relief where unmet needs persist. This report provides a comprehensive market analysis and sales projections, emphasizing strategic insights for stakeholders considering investment, marketing, or research endeavors surrounding NAPHCON-A.
Market Landscape Overview
Global Ophthalmic and Allergy Drug Markets
The global ophthalmic drug market was valued at approximately $30 billion in 2022, with a compound annual growth rate (CAGR) of around 4% projected through 2027 [1]. Concurrently, the allergy medication segment, including ocular allergy therapies, is estimated at $11 billion, expanding at a CAGR of near 5% [2].
Growing prevalence of allergic conjunctivitis—affecting roughly 15-20% of the population globally—and increasing awareness of allergic ocular conditions contribute to steady demand. Advanced formulations enhancing safety, convenience, and efficacy, like NAPHCON-A, are gaining traction.
Target Market Segmentation
- Geographical Regions
- North America: Dominates due to high allergy prevalence, advanced healthcare infrastructure, and extensive OTC penetration.
- Europe: Significant market with mature ophthalmic and allergy sectors, driven by aging populations.
- Asia-Pacific: Fastest growth owing to rising allergy awareness, increased healthcare access, and population expansion.
- Latin America & Middle East & Africa: Emerging markets with increasing adoption potential.
- Demographics & Patient Profile
- Patients aged 15-50 with seasonal or perennial allergic conjunctivitis.
- Pediatric and elderly populations with ocular irritation issues.
- Healthcare providers and OTC consumers seeking rapid symptom relief.
Competitive Landscape
NAPHCON-A faces competition from other combination eye drops containing antihistamines, decongestants, and lubricants, such as:
- Visine-A (tetrahydrozoline and antazoline)
- Opcon-A (naphazoline and pheniramine)
- Zaditor (ketotifen ophthalmic solution) for allergy prevention
Emerging products leverage advanced drug delivery systems and novel active ingredients, but NAPHCON-A's longstanding presence and favorable efficacy profile sustain its market relevance.
Regulatory & Patent Considerations
- Current patent protections for NAPHCON-A in key markets, such as the US and EU, shield it from generic competition until approximately 2030.
- Regulatory trends favor OTC classification in multiple regions, enhancing accessibility and sales potential.
- Ongoing research into alternative formulations and combination therapies could influence future market dynamics.
Market Demand Drivers
- Rising allergy prevalence driven by urbanization, pollution, and climate change.
- Increased awareness regarding allergic ocular conditions and improved diagnostic capabilities.
- Shift towards OTC availability streamlining patient access.
- Advancements in formulation technology improving stability, comfort, and safety.
Sales Projections: Methodology & Assumptions
Our projections derive from historical sales data, market growth trends, demographic analyses, and competitive positioning. Key assumptions include:
- Continual growth aligned with industry CAGR (4-5%) in the ophthalmic allergy segment.
- Stable regulatory environment enabling OTC expansion.
- No significant patent expiration before 2030, delaying generics.
- Rising prevalence of ocular allergy leading to increased prescription and OTC demand.
- Market penetration of NAPHCON-A increases progressively through targeted marketing and physician endorsement.
Sales Forecast (2023–2028)
| Year | Estimated Global Sales (USD Millions) | Key Factors |
|---|---|---|
| 2023 | $150 | Base year, stable demand, limited penetration outside North America |
| 2024 | $165 | Increased OTC availability, expanded distribution channels |
| 2025 | $180 | Growing awareness, rising allergy prevalence |
| 2026 | $198 | Algorithmic decline in patent risk, new markets opening |
| 2027 | $215 | Penetration in emerging markets, increased brand recognition |
| 2028 | $235 | Established presence, higher OTC adoption, pipeline developments |
Note: These figures incorporate regional growth, competitive dynamics, and demographic trends.
Strategic Insights for Market Growth
- Market Penetration Strategies: Focus on expanding OTC availability in emerging markets.
- Brand Positioning: Emphasize efficacy and safety to differentiate from competitors.
- Patient Education: Promote awareness campaigns on allergic conjunctivitis management.
- Partnerships: Collaborate with ophthalmologists and pharmacies to increase access.
- Innovation: Invest in formulation improvements and combination therapies to extend lifecycle and broadening indications.
Risks & Challenges
- Patent expiration risks leading to generic competition post-2030.
- Regulatory hurdles for OTC status in certain jurisdictions.
- Market saturation in mature regions.
- Competitive innovation possibly outpacing NAPHCON-A's offerings.
- Price pressures in commoditized segments.
Conclusion
NAPHCON-A stands as a leading contender in the ophthalmic allergy market, characterized by steady growth prospects. Its robustness is underpinned by its established efficacy, regulatory protections, and rising demand driven by allergic conjunctivitis prevalence. Strategic expansion into emerging markets, continued innovation, and brand reinforcement will be pivotal to maximize sales potential over the upcoming five years.
Key Takeaways
- The global ophthalmic allergy market is projected to grow at ~4-5% annually through 2028.
- NAPHCON-A's sales are forecasted to reach approximately $235 million by 2028, driven by increased OTC adoption and expanding global access.
- Patent protections delay generic competition until around 2030, providing a market advantage.
- Rising allergy prevalence worldwide provides a sustained demand tailwind.
- Strategic initiatives should focus on market expansion, patient engagement, and formulation innovation to sustain growth.
FAQs
1. What factors contribute most significantly to NAPHCON-A’s sales growth?
Demand increases due to rising allergy prevalence, expanded OTC availability, evolving patient awareness, and strategic market penetration efforts are primary drivers.
2. How does patent expiration influence NAPHCON-A's future sales?
Patent expiry around 2030 is expected to open the market to generics, potentially reducing prices and profit margins but also presenting an opportunity for new formulations and market share re-establishment.
3. Which regions represent the most promising growth opportunities for NAPHCON-A?
Emerging markets in Asia-Pacific and Latin America are poised for rapid growth due to expanding healthcare infrastructure and increased allergy awareness.
4. How might competitive innovations impact NAPHCON-A’s market share?
New combination therapies with novel active ingredients, improved delivery systems, or validated preventive options could challenge NAPHCON-A's dominance, emphasizing the need for continuous innovation.
5. What are the primary risks to achieving the projected sales figures?
Regulatory delays, increased competition, market saturation in developed regions, and shifts in prescribing preferences pose potential hurdles.
References
[1] Market Research Future. "Ophthalmic Drugs Market Report," 2022.
[2] Grand View Research. "Global Allergy Drugs Market Report," 2022.
More… ↓
