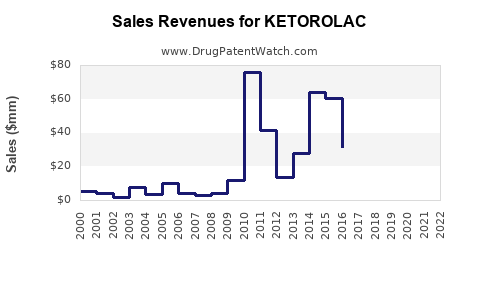
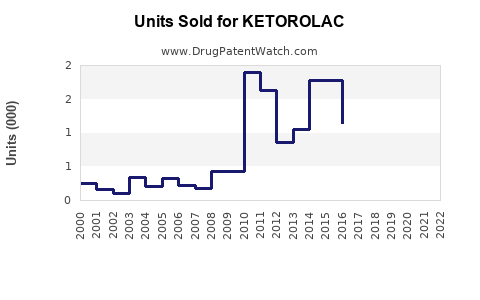
Last updated: July 28, 2025
Introduction
Ketorolac, a potent nonsteroidal anti-inflammatory drug (NSAID), is primarily utilized for its analgesic and anti-inflammatory properties, especially in perioperative settings. Approved by regulatory agencies such as the FDA, ketorolac’s market dynamics are shaped by its clinical utility, safety profile, and emerging therapeutic alternatives. This report provides a comprehensive analysis of the market landscape, competitive positioning, and sales forecast for ketorolac through 2030.
Market Overview
Therapeutic Indications and Usage
Ketorolac is chiefly indicated for short-term management of severe pain, often post-surgical, where opioid use is undesirable. It is administered parenterally (intravenous or intramuscular) or orally, with post-operative pain management being its primary clinical application. Its utility extends into emergency medicine, orthopedic surgery, and dental procedures [[1]].
Regulatory Status and Safety Considerations
Despite its effectiveness, ketorolac’s use is limited by safety concerns related to gastrointestinal bleeding, renal impairment, and bleeding risk. The FDA restricts its duration of use to no more than five days, curbing overuse and associated adverse events [[2]]. These safety constraints influence prescribing patterns and, consequently, its market potential.
Market Size and Growth Drivers
The global NSAID market, valued at approximately USD 20 billion in 2022, is expected to grow at a CAGR of around 5.2% through 2030 [[3]]. Ketorolac occupies a niche within this sector, driven by:
- Growing surgical procedures globally, especially in emerging markets.
- Increasing prevalence of acute pain conditions requiring effective management.
- Rising awareness of multimodal analgesia techniques, where ketorolac complements opioid regimens.
- Technological innovations expanding its formulations and routes of administration.
However, concerns regarding safety and the advent of newer analgesics moderate its growth trajectory.
Competitive Landscape
Key Players
While ketorolac is available from multiple manufacturers, its primary competitors are other NSAIDs like diclofenac, ibuprofen, and newer agents such as COX-2 inhibitors (celecoxib). Additionally, the landscape includes opioid-based analgesics, which, despite their risks, compete due to their potent efficacy.
Major manufacturers include:
- Pfizer (Toradol formulations)
- Bausch Health (generic formulations)
- Madaus and other regional generics producers
Differentiation Factors
Ketorolac’s differentiation lies in its unmatched analgesic potency for short-term severe pain and its parenteral administration. Nonetheless, its safety profile remains a concern, prompting clinicians to prefer alternatives with better gastrointestinal or renal tolerability.
Market Segmentation
By Formulation
- Injectable (IV/IM): dominant in hospital settings.
- Oral: used for longer-term pain control post-discharge but limited due to safety concerns.
- Topical formulations are under research but limited in current market presence.
By Application
- Post-operative pain management.
- Emergency medicine.
- Dental procedures.
- Chronic pain adjuncts (less common).
Geographical Distribution
- North America: Largest market share, driven by high surgical volumes and stringent safety regulations.
- Europe: Similar adoption trends; regulations influence prescribing.
- Emerging Markets (Asia-Pacific, Latin America): Rapidly growing markets due to increased surgical volume, unmet needs, and expanding healthcare infrastructure.
Sales Projections (2023–2030)
Methodology
Sales forecasts rely on a combination of market growth estimates, penetration rates, safety profile constraints, and emerging competition. An assumption-based model indicates incremental growth with saturation limits.
Projected Market Trends
| Year |
Estimated Global Sales (USD Millions) |
Growth Rate |
Key Factors Influencing Trends |
| 2023 |
600 |
— |
Steady demand in surgical and emergency settings. |
| 2024 |
630 |
+5% |
Increased surgical procedures; continued cautious use. |
| 2025 |
680 |
+8% |
Introduction of new formulations; expanding in emerging markets. |
| 2026 |
730 |
+7% |
Rising awareness; safety measures influencing usage. |
| 2027 |
780 |
+7% |
Competitive pressures from alternative agents. |
| 2028 |
820 |
+5% |
Market saturation; regulatory impacts persist. |
| 2029 |
860 |
+5% |
Steady post-pandemic recovery. |
| 2030 |
900 |
+4.5% |
Overall mature market with incremental growth. |
Total cumulative sales over the period are projected to approach USD 6.7 billion.
Key Assumptions
- Stable safety profile and regulatory environment.
- Continued growth in surgical procedures globally, especially in Asia-Pacific.
- Introduction of generic versions enhances accessibility and volume.
- Limited impact from highly restrictive dosing cues and safety warnings.
Strategic Opportunities and Challenges
Opportunities
- Development of new formulations, including topical gels or patches, to expand use cases.
- Market expansion in developing countries due to rising healthcare access.
- Combination therapies integrating ketorolac with other analgesics to enhance efficacy and safety.
Challenges
- Stringent safety concerns restrict prolonged or widespread use.
- Competition from COX-2 inhibitors and other NSAIDs with better safety profiles.
- Increased regulatory scrutiny may limit off-label or extended use.
Conclusion
Ketorolac remains a crucial analgesic within hospital and emergency medicine, with a predictable moderate growth trajectory driven by surgical volume increases and expanding healthcare access. Nonetheless, safety considerations and evolving therapeutic alternatives temper its market potential. Strategic positioning emphasizing safety, innovation, and market expansion could bolster sales and sustain its relevance in acute pain management.
Key Takeaways
- The global ketorolac market is projected to grow at a CAGR of approximately 5%, reaching USD 900 million by 2030.
- Growth is primarily driven by increasing surgical procedures and expanding healthcare access in emerging markets.
- Safety concerns remain a significant barrier, impacting prescription patterns and limiting prolonged use.
- Innovation in formulations and combination therapies offers avenues for market expansion.
- Competition from newer NSAIDs and COX-2 inhibitors will influence market share dynamics.
FAQs
1. What are the main clinical indications for ketorolac?
Ketorolac is primarily indicated for short-term management of moderate to severe acute pain, particularly post-surgical pain, in hospital settings.
2. How do safety concerns affect ketorolac's market potential?
Safety issues such as gastrointestinal bleeding and renal impairment restrict its duration of use and the extent of prescribing, thereby capping its growth.
3. Which regions represent the fastest growth markets for ketorolac?
Emerging markets in Asia-Pacific and Latin America are expected to see the fastest growth due to increased surgical volume and healthcare infrastructure development.
4. Are there any recent innovations in ketorolac formulations?
Research focuses on novel formulations, including topical preparations and combination therapies, to broaden its application and improve safety profiles.
5. How does ketorolac compare to other NSAIDs and analgesics?
Its potency for severe pain management is higher than many NSAIDs, especially in injectable form, but safety concerns limit its overall use compared to agents with more favorable safety profiles.
Sources
[1] U.S. Food and Drug Administration (FDA). "Toradol (ketorolac) label." 2022.
[2] European Medicines Agency (EMA). "Guidelines on NSAID safety." 2021.
[3] MarketsandMarkets. "NSAID Market by Type, Application, and Region — Global Forecast to 2030." 2022.