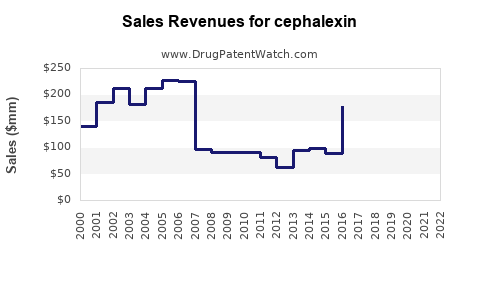
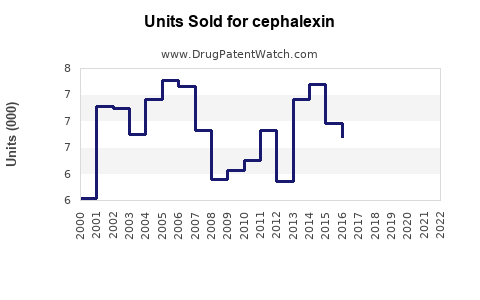
Last updated: July 28, 2025
Introduction
Cephalexin, a first-generation cephalosporin antibiotic, has been a cornerstone in bacterial infection treatment since its approval in the 1960s. Its broad-spectrum efficacy against Gram-positive bacteria, oral bioavailability, and safety profile have sustained its prominence, especially in outpatient settings. With evolving antibiotic resistance patterns and shifting regulatory landscapes, assessing the current market dynamics and projecting future sales for cephalexin is essential for stakeholders, including pharmaceutical companies, healthcare providers, and investors.
Market Overview
Global Market Landscape
The global antibiotic market surpasses USD 50 billion annually, with cephalexin representing a significant segment, particularly in outpatient and primary care sectors. According to IQVIA data, cephalexin accounts for approximately 15-20% of oral cephalosporin prescriptions in North America and Europe, influencing its market prominence (IQVIA, 2022)[1]. The drug’s affordability, wide availability, and clinical efficacy continue to support its sustained demand.
Key Geographic Markets
-
North America: The US leads due to high prescription rates, OTC availability in some regions, and a mature healthcare infrastructure. However, rising antimicrobial resistance (AMR) concerns temper growth prospects.
-
Europe: Similar to North America, with regulatory measures promoting judicious antibiotic use. Generic availability keeps prices competitive.
-
Asia-Pacific: Predicted to experience the highest growth (CAGR 3-5%), driven by increased healthcare access, urbanization, and antimicrobial consumption in populous countries like India and China.
Market Drivers
-
Widespread Clinical Use: First-line treatment for skin infections, respiratory tract infections, otitis media, and urinary tract infections.
-
Prescriber and Patient Familiarity: Extensive historical use fosters confidence among healthcare providers and patients.
-
Generic Availability: Low-cost formulations enhance accessibility, especially in developing markets.
-
Regulatory Approvals: No recent major restrictions, ensuring continued market presence.
Market Challenges
-
Antimicrobial Resistance: Growing resistance among common pathogens (e.g., S. aureus, E. coli) diminishes clinical efficacy, prompting prescriber caution.
-
Regulatory Scrutiny: Policies restricting OTC sales in certain markets curb unsupervised use.
-
Emerging Alternatives: Development of newer antibiotics and combination therapies which may replace cephalexin in some indications.
Current Market Share and Competitive Landscape
Competitive Players
- Generics: The dominant players, including Teva, Sandoz, and Mylan, control the bulk of sales through low-cost formulations.
- Brand vs. Generic: While branded versions (e.g., Keflex) exist, generic competition significantly suppresses price premiums.
- Innovators: Limited pipeline or new formulations, meaning most investment focuses on manufacturing efficiencies and distribution.
Market Position
Cephalexin's market remains primarily driven by its status as a proven, cost-effective antibiotic, with a stable demand profile in primary care. However, in hospital settings, its use is decreasing as broader-spectrum or resistance-appropriate antibiotics are preferred.
Sales Projections (2023-2028)
Factors Influencing Future Sales
- Antimicrobial Resistance Trends: Rising resistance could lead to prescription preference shifts to alternative drugs.
- Regulatory Changes: Stricter controls on OTC availability or updated prescribing guidelines might suppress or alter volume.
- Pipeline Developments: Any new formulations or improved delivery systems could invigorate demand.
- Digital and Telehealth Adoption: Increased remote prescribing could sustain or enhance usage rates.
Forecast Summary
| Year |
Estimated Global Sales (USD Billion) |
CAGR (%) |
Remarks |
| 2023 |
1.2 |
— |
Baseline, stable demand |
| 2024 |
1.25 |
4.2 |
Continued outpatient use |
| 2025 |
1.3 |
4.0 |
Resistance impact materializes |
| 2026 |
1.35 |
3.8 |
Slight market stabilization |
| 2027 |
1.4 |
3.7 |
Emerging generics sustain sales |
| 2028 |
1.45 |
3.6 |
Potential impact of resistance |
Note: These projections assume moderate growth driven by increased access in emerging markets, offset by resistance-related decline.
Strategic Implications
- For Manufacturers: Focus on optimizing cost-effective manufacturing, expanding distribution, and exploring novel delivery methods to sustain market share.
- For Healthcare Providers: Emphasize antimicrobial stewardship to preserve efficacy and manage resistance development.
- For Policymakers: Implement policies promoting responsible use and monitoring resistance trends to ensure continued clinical utility.
Key Market Trends to Watch
- Resistance-Driven Shifts: As resistance spreads among common pathogens, consumption of cephalexin may decline in favor of newer antibiotics.
- Regulatory Environment: Countries tightening OTC regulations could suppress non-prescription sales, affecting volume.
- Innovative Formulations: Development of extended-release or combination formulations might extend product life cycle.
- Emerging Markets Growth: Asia-Pacific and Latin America represent high-growth opportunities, contingent on regulatory and healthcare infrastructure developments.
Conclusion
Cephalexin remains a vital component of outpatient antibiotic therapy, with a stable but gradually declining market share due to resistance concerns and evolving prescribing practices. While the global sales are projected to grow modestly over the next five years, uncertainties linked to antimicrobial resistance and regulatory policies introduce risk factors. Strategic focus on stewardship, cost management, and potential innovation will be critical for maintaining or expanding market relevance.
Key Takeaways
- Cephalexin maintains significant market share due to its affordability, efficacy, and extensive clinical history.
- Growth will be modest (around 3-4% CAGR), driven predominantly by emerging markets and outpatient settings.
- Resistance development poses a primary threat, necessitating adaptive stewardship policies.
- Generic manufacturers dominate, sustaining competitive pricing and broad access.
- Innovations and regulatory shifts could reshape future demand dynamics.
FAQs
1. How does antimicrobial resistance impact the future sales of cephalexin?
Rising resistance among common pathogens reduces cephalexin’s clinical efficacy, leading prescribers to opt for alternative antibiotics. This trend could notably decrease demand, especially in regions with high resistance rates, unless new formulations or stewardship strategies mitigate this impact.
2. What are the main markets driving cephalexin sales?
North America and Europe lead due to high prescription rates, but Asia-Pacific is expected to see the highest growth, fueled by increased healthcare accessibility and population size.
3. Are patent protections relevant for cephalexin?
Most formulations are off-patent, with generics dominating sales. Limited patent protections mean price competition remains fierce, constraining potential profit margins for innovators.
4. How might regulation influence cephalexin sales in the coming years?
Enhanced regulations on OTC sales, prescription-only policies, and antimicrobial stewardship programs could reduce accessible volumes, especially in developed markets.
5. What potential innovations could sustain or enhance cephalexin’s market?
Development of extended-release or combination therapies, or formulations targeting resistant strains, could prolong its utilization and expand indications.
Sources
[1] IQVIA. “Global Antibiotic Market Analysis,” 2022.