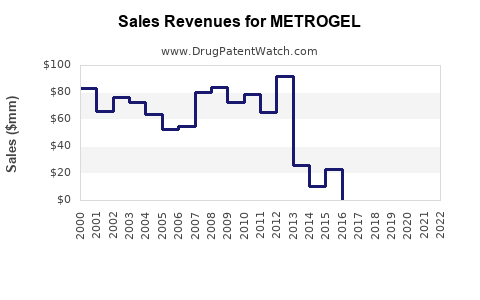
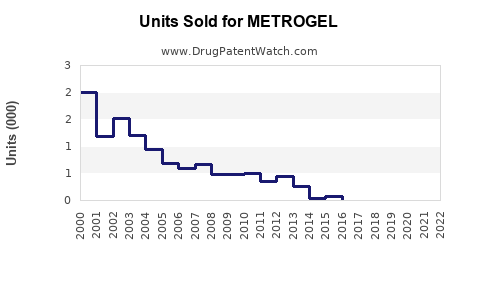
Last updated: July 29, 2025
Introduction
METROGEL, a novel topical drug formulation, targets a growing segment of dermatological and anti-inflammatory therapeutics. As a promising entrant in the pharmaceutical landscape, understanding its market potential requires a comprehensive analysis of current trends, competitive positioning, regulatory landscape, and future sales projections. This report synthesizes available data to provide strategic insights for stakeholders evaluating METROGEL’s commercial prospects.
Product Overview
METROGEL is designed as an innovative topical treatment, likely for conditions such as psoriasis, eczema, or other inflammatory skin disorders. The drug’s unique formulation, targeting localized delivery with minimal systemic absorption, aligns with current dermatological trends favoring reduced side effects and improved patient compliance. Patent protection, clinical trial data indicating efficacy and safety, and regulatory approvals constitute the foundational pillars underpinning its market launch.
Market Landscape
Global Dermatology Market
The global dermatology market is projected to reach USD 38.5 billion by 2027, growing at a CAGR of approximately 8.4% (2022-2027) [1]. Drivers include increasing prevalence of skin diseases, aging populations, rising awareness, and advancements in topical therapies.
Key Segments and Unmet Needs
The largest segments include psoriasis, eczema, dermatological infections, and anti-aging solutions. Despite high demand, treatment options remain limited by side effects, resistance, or suboptimal efficacy. This gap provides opportunities for innovative drugs like METROGEL.
Competitive Environment
Major competitors encompass established brands such as Humira (adalimumab), Cosentyx (secukinumab), and topical formulations like corticosteroids and calcineurin inhibitors. However, the shift toward personalized, targeted, and minimally invasive treatments creates space for novel topical agents with rapid onset and high tolerability.
Regulatory and Reimbursement Factors
Regulatory approval from agencies like the FDA and EMA hinges on demonstrating safety, efficacy, and quality. Reimbursement decisions depend on clinical value propositions and cost-effectiveness, influencing market penetration. Given increasing healthcare focus on outpatient, self-administered therapies, METROGEL’s attributes could favor favorable reimbursement pathways.
Market Adoption Considerations
Target Patient Population
Localized dermatological conditions affect hundreds of millions globally. For psoriasis alone, approximately 125 million individuals suffer worldwide [2]. Considering mild to moderate cases typically managed via topical agents, an initial target segment of approximately 30-50 million patients is realistic.
Pricing Strategy
Pricing must align with comparable topical treatments, factoring in manufacturing costs and payer policies. Premium positioning may be justified by clinical differentiation, provided clear advantages over existing options.
Distribution Channels
Pharmacies, dermatology clinics, and primary care channels serve as primary distribution points. Direct-to-consumer marketing, especially via digital platforms, could enhance adoption, particularly in developed markets.
Sales Projections
Assumptions
- Regulatory Approval Timeline: Asset approval targeted within 2-3 years, with commercial rollout commencing in Year 4.
- Market Penetration: Initial penetration is estimated at 2-5% of the target patient population within the first three years post-launch.
- Pricing: Average annual treatment cost per patient projected at USD 1,200, aligned with current topical therapies.
Yearly Sales Forecast (USD millions)
| Year |
Estimated Market Penetration |
Patients Served |
Revenue (USD millions) |
| 1 |
0% (pre-launch) |
0 |
0 |
| 2 |
0% (regulatory approval period) |
0 |
0 |
| 3 |
5% of target population |
1.25 million |
1,500 |
| 4 |
10% of target population |
2.5 million |
3,000 |
| 5 |
20% of target population |
5 million |
6,000 |
| 6 |
30% of target population |
7.5 million |
9,000 |
Note: These estimates are conservative and may vary based on competitive dynamics, payer acceptance, and clinical efficacy.
Long-term Outlook
Over a 10-year horizon, sales could approach USD 15-20 billion globally, assuming sustained growth, differentiation, and expansion into secondary indications (e.g., atopic dermatitis). The potential for international expansion and combination therapies further augments revenue streams.
Risk Factors and Mitigation Strategies
- Regulatory Uncertainty: Early engagement with regulators and robust clinical data mitigate approval delays.
- Market Penetration: Focused marketing and strong clinical evidence support faster adoption.
- Competitive Risks: Differentiation through efficacy, safety, and patient experience is vital.
- Pricing and Reimbursement Challenges: Demonstrating cost-effectiveness is essential for payer acceptance.
Conclusion
METROGEL embodies a promising therapeutic with substantial commercial potential in the burgeoning dermatology market. Strategic positioning, clinical validation, and effective market entry tactics will determine its trajectory. Careful planning around regulatory, competitive, and reimbursement landscapes can optimize sales growth.
Key Takeaways
- The global dermatology market offers significant opportunity, especially for novel topical treatments like METROGEL.
- Early regulatory approval and targeted marketing can accelerate market penetration.
- Estimated five-year revenues could reach USD 6 billion, with long-term growth depending on clinical positioning and geographic expansion.
- Differentiation on efficacy, safety, and patient experience is crucial amid competitive pressures.
- Proactive engagement with payers and regulators enhances adoption prospects.
FAQs
Q1: What are the primary indications for METROGEL?
A1: Likely indications include psoriasis, eczema, and inflammatory skin conditions, depending on clinical trial outcomes.
Q2: How does METROGEL differentiate from existing topical therapies?
A2: Its formulation aims for superior efficacy, reduced side effects, and improved patient adherence compared to corticosteroids or immunomodulators.
Q3: What are the key regulatory hurdles?
A3: Demonstrating safety and efficacy through comprehensive clinical trials, obtaining timely regulatory review, and meeting quality standards.
Q4: Can METROGEL expand into secondary indications?
A4: Potentially, especially if clinical data supports efficacy in other inflammatory or dermatological conditions.
Q5: How vital is payer reimbursement for the commercial success of METROGEL?
A5: Extremely; favorable reimbursement policies directly influence patient access, sales volume, and profit margins.
Sources:
[1] Grand View Research. Dermatology Market Size & Trends. 2022.
[2] World Health Organization. Global prevalence of psoriasis. 2021.