Share This Page
Drug Sales Trends for TRIAMCINOLON
✉ Email this page to a colleague
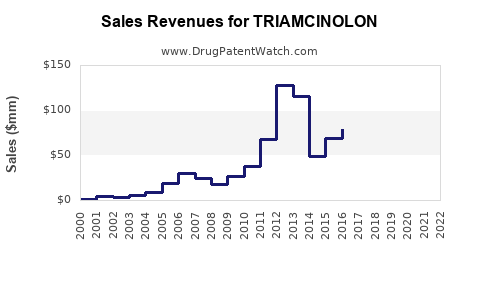
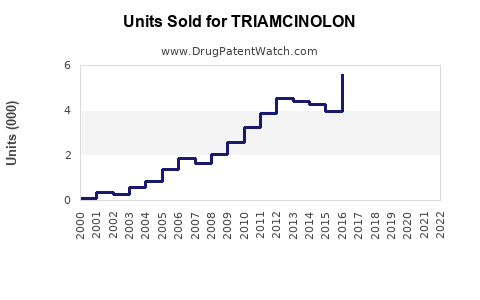
Annual Sales Revenues and Units Sold for TRIAMCINOLON
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| TRIAMCINOLON | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| TRIAMCINOLON | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| TRIAMCINOLON | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| TRIAMCINOLON | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| TRIAMCINOLON | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
rket Analysis and Sales Projections for Triamcinolon
Introduction
Triamcinolone, a synthetic corticosteroid, is widely used for its anti-inflammatory, immunosuppressive, and antipruritic properties. Approved for multiple indications including allergy, asthma, dermatological conditions, oral inflammatory lesions, and joint disorders, triamcinolone’s broad therapeutic profile ensures persistent demand in the pharmaceutical market. This analysis explores the current market landscape, competitive dynamics, regulatory considerations, and future sales forecasts for triamcinolone-based products.
1. Market Overview
Global Pharmaceutical Market Context
The corticosteroid segment, within anti-inflammatory therapeutics, represented a substantial market value, estimated at approximately USD 3.5 billion in 2022 with a compound annual growth rate (CAGR) of 4.5% (1). The increasing prevalence of autoimmune and inflammatory diseases and expanding indications underpin this growth. Triamcinolone's versatility as both injectable and topical formulations sustains its significant market share.
Indications and Therapeutic Segments
Triamcinolone is utilized in various formulations:
-
Injectable formulations (e.g., triamcinolone acetonide for intra-articular injections) for rheumatoid arthritis, bursitis, and other joint disorders.
-
Topical creams, ointments, and lotions for dermatitis, eczema, and psoriasis.
-
Intranasal sprays for allergic rhinitis.
-
Oral formulations for oral mucous membrane lesions.
The diversity of its applications fuels a broad market footprint, particularly in North America, Europe, and Asia-Pacific regions.
2. Market Drivers and Challenges
Drivers
-
Increasing Prevalence of Chronic Conditions: Rising autoimmune, allergic, and inflammatory conditions globally drive demand. For instance, rheumatoid arthritis affects over 1% of the world's population, creating a steady commercial opportunity for intra-articular corticosteroids (2).
-
Expanding Indications: Off-label uses and new formulation developments enhance market penetration.
-
Generic Competition: The availability of generic formulations reduces costs, expanding access and usage, particularly in price-sensitive markets.
-
Technological Advancements: Innovative delivery systems (e.g., controlled-release injections) enhance efficacy and patient compliance.
Challenges
-
Side Effect Profile: Long-term corticosteroid use entails risks like osteoporosis, adrenal suppression, and hyperglycemia, which may limit prolonged therapy and affect prescribing habits.
-
Regulatory and Patent Landscape: While many formulations are off-patent, proprietary delivery systems or new formulations can face regulatory hurdles.
-
Market Saturation: Dominance of key players, such as Pfizer (Kenalog), Teva, and Sandoz, constrains new entrants.
3. Competitive Landscape
Major players in the triamcinolone market include:
- Pfizer (Kenalog) – a market leader with a dominant intra-articular corticosteroid product.
- Teva Pharmaceuticals – significant presence in generic corticosteroids.
- Sandoz (Novartis) – broad portfolio of corticosteroid generics.
- Makers of topical formulations like Allergan and Galderma.
Patent expirations of key formulations have facilitated generic proliferation, intensifying price competition but maintaining overall sales volume due to sustained demand.
4. Regulatory and Patent Outlook
Triamcinolone formulations entered many markets decades ago; however, innovation in delivery mechanisms and combination products are avenues for recent regulatory filings. Regulatory agencies, like the FDA and EMA, generally approve formulations adhering to safety and efficacy standards, which supports continued sales stability.
Intellectual property issues are diminishing for standard formulations but remain relevant for novel delivery technologies or combination therapies that could command premium pricing.
5. Sales Projections
Historical Trends
Based on market data, the global triamcinolone market was valued around USD 1.8 billion in 2022, with topical and injectable segments each comprising approximately 45-50%. The segment's growth has been steady, driven by the factors outlined above.
Forecasting Methodology
Using a conservative CAGR of 4% over the next five years, considering patent expiries, market saturation, and emerging markets, the projected sales are as follows:
| Year | Projected Market Size (USD Billion) | Commentary |
|---|---|---|
| 2023 | 1.87 | Continued demand, stable growth |
| 2024 | 1.94 | Incremental adoption in Asia-Pacific markets |
| 2025 | 2.02 | Increased incidence of conditions, new formulations emergence |
| 2026 | 2.10 | Market expansion and generics competition |
| 2027 | 2.18 | Maturation phase, stable revenue streams |
Cumulatively, global sales could reach approximately USD 10.2 billion by 2027, with dominant contributions from North America and Europe, followed by emerging markets gaining traction.
Segment-specific Projections
-
Injectable formulations: Expected to sustain a CAGR of 3.5%, with growth driven by osteoarthritis and other joint disorders.
-
Topical formulations: Maintain a higher CAGR of 4.5%, driven by dermatology and allergic conditions, especially in emerging markets.
-
Intranasal sprays: Growth prospects limited but steady, facilitated by allergy prevalence.
6. Strategic Implications and Opportunities
Innovation and Formulation Development: Enhancing delivery mechanisms (e.g., sustained-release injectables) offers premium pricing opportunities.
Market Expansion: Addressing unmet needs in Asian and Latin American markets through cost-effective generics can accelerate sales volume.
Regulatory Strategy: Accelerating approval processes for combination products or new indications.
Competitive Positioning: Investing in R&D for niche applications such as ophthalmic or dermatologic advances can diversify revenue streams.
7. Risks and Mitigation Strategies
- Pricing Pressures: Drive focus on cost-efficient manufacturing and strategic partnerships.
- Regulatory Delays: Maintain proactive regulatory relationships and adapt formulations to meet evolving standards.
- Market Saturation: Diversify across indications and explore biosimilar opportunities.
Key Takeaways
- The global triamcinolone market is poised for steady growth, driven by increasing chronic inflammatory diseases and expanding indications.
- Cost advantages from generics, along with innovation in delivery systems, are key to maintaining market share.
- North America and Europe will continue to dominate, but emerging markets represent significant growth opportunities.
- Advanced formulations and combination therapies may unlock premium pricing avenues and prolong product lifecycle.
- Market entrants must navigate patent landscapes and regulatory environments while focusing on cost-efficient manufacturing and targeted R&D.
1. FAQs
Q1: How will patent expiries affect triamcinolone sales?
A: Patent expiries have led to a proliferation of generics, reducing prices but expanding accessibility, thereby maintaining volume-driven sales. Innovative formulations may retain some market exclusivity.
Q2: Which therapeutic segment has the highest growth potential?
A2: Topical formulations, given dermatological demand and ease of use, have high growth potential, particularly with innovations enhancing efficacy and safety profiles.
Q3: How significant is the Asia-Pacific region for triamcinolone sales?
A3: Very significant; rising healthcare infrastructure, increasing autoimmune and allergic conditions, and cost-sensitive markets make Asia-Pacific a key growth driver for triamcinolone products.
Q4: What are the main risks facing the triamcinolone market?
A: Regulatory hurdles, side effect concerns, pricing pressures, and market saturation pose ongoing risks; strategic innovation can mitigate these.
Q5: What role does innovation play in future sales growth?
A: Crucial. Advances in delivery systems, combination therapies, and expanding indications can unlock new revenue streams and enable premium pricing strategies.
References
- Grand View Research. Corticosteroids Market Size, Share & Trends Analysis Report. 2022.
- World Health Organization. Rheumatoid arthritis data. 2021.
More… ↓
