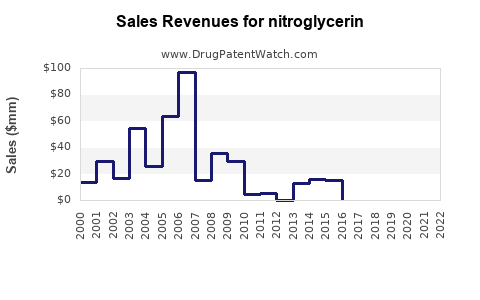
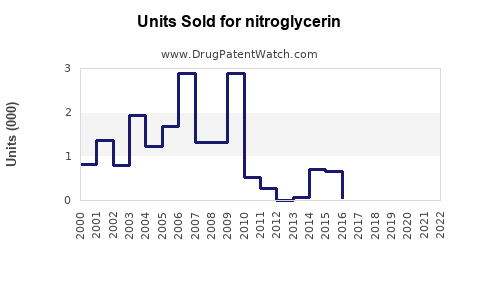
Last updated: July 27, 2025
Introduction
Nitroglycerin, a potent vasoactive medication primarily used for angina pectoris and acute coronary syndrome, remains a cornerstone in cardiovascular therapeutics. With its longstanding history, the drug continues to demonstrate sustained demand, driven by its proven efficacy and widespread clinical use. This analysis evaluates the current market landscape, competitive environment, regulatory factors, and future sales projections, providing critical insights for stakeholders in the pharmaceutical industry.
Market Overview
Historical Context and Clinical Significance
Introduced in the late 19th century, nitroglycerin’s cardioprotective roles have cemented its place in cardiac care. Its mechanism—vasodilation via nitric oxide release—improves blood flow, alleviating angina symptoms. It is available across multiple formulations: sublingual tablets, patches, ointments, intravenous preparations, and transdermal patches.
Market Size and Trends
The global cardiovascular drugs market, including nitrates like nitroglycerin, was valued at approximately USD 50 billion in 2022, with nitrates comprising a significant segment due to the high prevalence of ischemic heart disease. Nitroglycerin specifically accounts for an estimated USD 2–3 billion of this market.
The growth trajectory is influenced by factors such as rising cardiovascular disease (CVD) prevalence, aging populations, expanding healthcare infrastructure in emerging markets, and continued physician reliance on established therapies. The segment has experienced moderate growth of about 3–5% annually over the past five years, driven largely by North America and Europe, with emerging markets showing increasing adoption.
Regulatory and Patent Landscape
Nitroglycerin is off-patent in most jurisdictions, classified as a generic medication. This openness has led to multiple manufacturers offering variant formulations, intensifying price competition but also catalyzing market volume growth. Regulatory standards, such as FDA approval and EMA compliance, ensure quality but do not impede the entry of generics.
Market Drivers and Barriers
Key Drivers
-
High Prevalence of Cardiovascular Diseases: According to the World Health Organization (WHO), CVD accounts for roughly 17.9 million deaths annually, fueling sustained demand for anti-anginal drugs like nitroglycerin.
-
Cost-Effectiveness and Established Efficacy: Its longstanding clinical use fosters physician confidence, especially in acute settings.
-
Expansion into Developing Markets: Increasing healthcare access and awareness drive consumption in regions with rising CVD burden.
Market Barriers
-
Availability of Alternative Therapies: Newer drug classes (e.g., ranolazine, ivabradine) and combination therapies may limit uptake in some contexts.
-
Formulation Limitations: Short shelf life for certain formulations (e.g., sublingual tablets) and the need for proper storage pose logistical constraints.
-
Regulatory Hurdles and Reimbursement Policies: Variability across markets affects sales potential, especially in payor-restricted environments.
Competitive Landscape
Major Players
While many manufacturers produce nitroglycerin formulations, the market is characterized by a few dominant generic suppliers, such as Pfizer, Mylan, and Teva Pharmaceuticals. Brands like Nitro-Dur (transdermal patches) and Nitrostat (sublingual tablets) hold significant market share due to brand recognition and healthcare provider familiarity.
Market Dynamics
-
Generic Penetration: High generic availability keeps prices competitive but limits margins for manufacturers.
-
Innovation & Formulation Development: Slight formulation improvements, such as extended-release patches, aim to improve patient compliance and clinical outcomes.
-
Distribution Channels: Hospitals, clinics, and pharmacy chains are primary channels, with online pharmacies gaining traction in certain regions.
Regulatory Considerations
As a mature, off-patent drug, nitroglycerin's regulatory pathway centers around maintaining quality standards rather than new approvals. However, geographic-specific regulations for manufacturing, labeling, and distribution influence market access. Additionally, medication safety concerns, such as tolerance with long-term use, necessitate ongoing pharmacovigilance.
Sales Projections (2023–2033)
Methodology
Projection models incorporate epidemiological data, current market trends, demographic shifts, and potential growth in emerging markets. Assumptions include steady adoption based on CVD prevalence, continued generic competition, and minimal impact from disruptive innovations.
Forecast Highlights
-
2023–2027: The annual sales are expected to grow at a compound annual growth rate (CAGR) of approximately 2.5%, reaching approximately USD 3.1 billion by 2027. Growth will be driven primarily by increased prevalence in aging populations and expansion into emerging markets.
-
2028–2033: From 2028 onward, sales are projected to stabilize around USD 3.2–3.4 billion. Slight declines may occur due to competition from alternative treatments and changing clinical practices favoring newer agents. However, the high clinical reliance on nitroglycerin ensures a steady baseline demand.
Emerging Market Opportunities
As healthcare infrastructure improves and awareness of CVD management rises, markets in Asia-Pacific, Latin America, and Africa are expected to contribute an additional 15–20% of total sales growth over the projection period.
Innovation and Future Outlook
Although the drug is generic, ongoing research aims to optimize formulations—such as transdermal patches with extended duration, improved stability, and reduced tolerance development—to enhance patient adherence and outcomes. Furthermore, combination therapies incorporating nitroglycerin are under clinical investigation, potentially expanding its therapeutic scope.
Advancements in drug delivery systems, including nanotechnology-based formulations, may rejuvenate interest and extend market relevance. Regulatory agencies are also emphasizing personalized medicine approaches, which could influence prescribing patterns.
Conclusion
Nitroglycerin's market remains robust, bolstered by its long-standing efficacy and widespread clinical use. Despite intense price competition and the advent of alternative therapies, its role in acute and chronic anginal treatment sustains sales. The most significant growth prospects lie in emerging markets, driven by epidemiological trends and expanding healthcare coverage.
Sustained innovation in formulation, along with strategic market expansion, can further capitalize on this enduring demand. Stakeholders should monitor regulatory developments, epidemiological shifts, and technological advances to optimize market positioning over the coming decade.
Key Takeaways
-
Stable Demand: Nitroglycerin’s proven efficacy ensures consistent demand, with projected global sales reaching around USD 3.4 billion by 2033.
-
Market Growth Drivers: Rising CVD prevalence and increased healthcare infrastructure in emerging markets underpin growth potential.
-
Competitive Dynamics: The market is heavily commoditized, emphasizing cost-efficiency and formulation innovations to differentiate offerings.
-
Regulatory Environment: Low barriers due to being off-patent facilitate widespread manufacturing but necessitate compliance with quality standards.
-
Innovation Opportunities: Formulation enhancements and combination therapies offer avenues to extend product life cycles and expand clinical applications.
FAQs
1. How does the aging population influence nitroglycerin sales?
An aging demographic increases the prevalence of ischemic heart disease, thereby elevating demand for nitroglycerin in both acute and chronic management of angina, directly boosting sales potential.
2. What challenges do generic formulations face in maintaining market share?
Intense price competition, arbitrary formulary decisions, and the entry of new delivery systems challenge incumbents. Maintaining differentiation through formulation improvements or delivery innovations is critical.
3. Are there emerging therapies that could replace nitroglycerin?
While newer agents (e.g., ranolazine, ivabradine) are gaining popularity, none have yet supplanted nitroglycerin’s role in acute angina management due to its rapid action and proven efficacy.
4. How might regulatory changes impact nitroglycerin market growth?
Stringent safety and quality standards can increase manufacturing costs but generally do not impede market access due to the drug’s established credibility. Enhanced regulatory oversight may, however, influence formulations and labeling.
5. What opportunities exist for manufacturers in emerging markets?
Expanding production, tailoring formulations to regional needs, and establishing distribution networks present significant growth opportunities amid rising CVD burdens in these regions.
References
[1] World Health Organization. (2021). "Cardiovascular Diseases (CVDs)."
[2] Grand View Research. (2022). "Global Cardiovascular Drugs Market Size, Share & Trends."
[3] U.S. Food & Drug Administration. (2022). "Off-Patent Drugs & Market Dynamics."
[4] Statista. (2023). "Global Sales of Nitrates and Antanginal Drugs."
[5] European Medicines Agency. (2021). "Regulatory Guidelines for Cardiology Drugs."