Share This Page
Drug Sales Trends for FLUDROCORT
✉ Email this page to a colleague
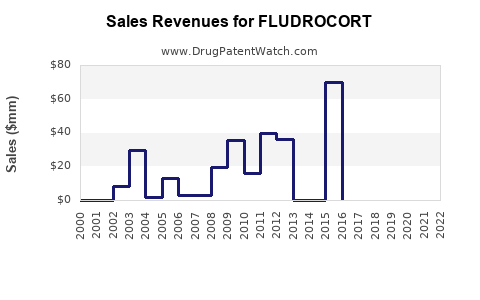
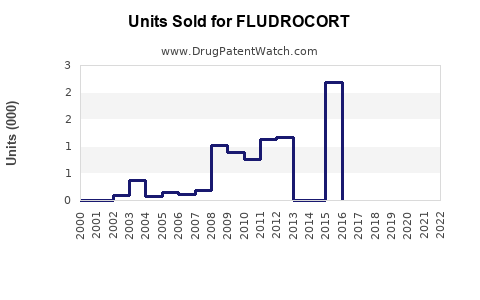
Annual Sales Revenues and Units Sold for FLUDROCORT
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| FLUDROCORT | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| FLUDROCORT | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| FLUDROCORT | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| FLUDROCORT | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| FLUDROCORT | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| FLUDROCORT | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for Fludrocortisone
Introduction
Fludrocortisone stands as a synthetic corticosteroid primarily prescribed for mineralocorticoid replacement therapy in conditions like Addison’s disease, primary or secondary adrenal insufficiency, and certain renal salt-wasting disorders. Its unique role in managing electrolyte balance and blood pressure positions it within a specialized segment of endocrinology and nephrology. As healthcare landscapes evolve, so do the opportunities for Fludrocortisone’s market expansion, driven by demographic shifts, clinical guidelines, and pharmaceutical innovation.
Market Overview
Global Therapeutic Area and Demand Drivers
The corticosteroid market, valued at approximately USD 14 billion in 2022, encompasses drugs used for inflammation, autoimmune diseases, and adrenal disorders. Fludrocortisone occupies a niche within this space, with its demand tied closely to the prevalence of adrenal disorders. Key demand drivers include:
- Aging Population: The global elderly demographic is expanding rapidly, increasing the incidence of adrenal insufficiency, often related to age-associated adrenal decline or secondary causes [1].
- Enhanced Diagnostic Precision: Better screening and diagnosis of adrenal disorders lead to increased prescriptions of mineralocorticoid therapy.
- Advances in Disease Management: Improved patient management protocols favor the continued use of Fludrocortisone as a first-line therapy.
Geographical Market Dynamics
The largest markets for Fludrocortisone are:
- North America: Driven by well-established healthcare infrastructure, high disease awareness, and robust pharmaceutical sales mechanisms.
- Europe: Similar to North America with high prevalence rates and advanced healthcare systems.
- Asia-Pacific: Anticipated rapid growth due to rising prevalence, increased healthcare investments, and expanding pharmaceutical manufacturing.
Competitive Landscape
While Fludrocortisone has been available for decades, it faces competition from newer corticosteroids with differing potency profiles or alternative therapeutic approaches such as mineralocorticoid receptor antagonists. However, its cost-effectiveness and established efficacy preserve its market relevance, especially in resource-constrained settings.
Sales Projections (2023–2030)
Current Market Size and Trends
In 2022, the global Fludrocortisone market was estimated at USD 250–300 million, primarily driven by prescription volume. The market growth is modest, averaging around 3-5% CAGR over recent years, influenced by steady demand in hormone replacement therapy and limited scope for off-label use.
Projected Growth Factors
- Increased Diagnosis of Adrenal Insufficiency: Accelerating diagnosis rates could augment demand, especially in developing regions adopting Western diagnostic standards.
- Expanded Formulations: Introduction of new formulations (e.g., sustained-release) could enhance patient compliance and sales volume.
- Regulatory Approvals: Efforts to expand indications or streamline approval processes can bolster market size.
Forecast Overview
Based on current trends, by 2030, the Fludrocortisone market could reach USD 400–500 million, driven by population aging, expanded indications, and increased healthcare access globally. The CAGR is projected around 4-6%, with regional variances—faster in Asia-Pacific and slower in mature markets due to market saturation.
Market Challenges and Opportunities
Challenges
- Limited Off-label Applications: Restricted to primarily adrenal deficiency, limiting diversification.
- Generic Competition: Widespread availability as a generic drug suppresses prices and profit margins.
- Regulatory Hurdles: Stringent approval processes in certain regions impede new formulations or indications.
Opportunities
- New Delivery Technologies: Development of sustained-release or transdermal formulations could elevate adherence.
- Biomarker-Based Personalized Therapy: Tailoring dosage regimens might improve efficacy and expand usage.
- Emerging Markets: Market penetration in underdeveloped countries offers untapped growth potential.
Conclusion
The Fludrocortisone market exhibits steady, moderate growth, sustained by its vital role in adrenal hormone replacement therapies. While facing competitive pressures from generics and alternative therapies, strategic innovations and demographic trends underpin a positive long-term outlook. Stakeholders should prioritize regional expansion, formulation innovation, and physiological research to capitalize on emerging opportunities.
Key Takeaways
- The global Fludrocortisone market is poised to reach USD 400–500 million by 2030, with a CAGR of approximately 4-6%.
- North America and Europe dominate current sales; however, Asia-Pacific offers significant growth potential.
- Market growth hinges on increased diagnosis, approval of new formulations, and expanding healthcare infrastructure.
- The drug’s core application in adrenal insufficiency ensures steady demand, but competition from generics pressures pricing.
- Innovation in drug delivery and targeted therapy can unlock additional market segments and improve patient outcomes.
FAQs
1. What are the primary factors influencing Fludrocortisone sales?
Demand is primarily influenced by the prevalence of adrenal insufficiency, advancements in diagnostic capabilities, healthcare access, and formulary approvals. Demographic shifts toward aging populations generally increase the need for mineralocorticoid replacement therapy.
2. How does regional healthcare infrastructure impact Fludrocortisone sales?
Regions with advanced healthcare systems, such as North America and Europe, facilitate higher prescription rates due to better diagnosis and treatment adherence. Emerging markets with expanding healthcare infrastructure are expected to contribute increasingly to demand growth.
3. What are the main competitive threats to Fludrocortisone?
Intense competition from generic manufacturers constrains pricing power. Additionally, alternative therapies such as mineralocorticoid receptor antagonists and newer synthetic corticosteroids pose substitution risks.
4. Are there any upcoming formulations or formulations innovations for Fludrocortisone?
While currently available mainly as oral tablets, development of sustained-release formulations and transdermal patches could improve adherence and expand its marketability, especially for chronic management.
5. How might regulatory changes influence Fludrocortisone market growth?
Streamlined approval processes, expanded indications, and inclusion in clinical guidelines can facilitate market penetration. Conversely, regulatory hurdles or restrictions on off-label use could dampen growth prospects.
References
[1] World Health Organization. (2022). "Aging and health: A global overview."
[2] Grand View Research. (2022). "Corticosteroids Market Size, Share & Trends."
[3] Pfizer. (2021). "Drug Use in Endocrinology: A Market Perspective."
More… ↓
