Last updated: July 27, 2025
Introduction
Levothyroxine, a synthetic form of the thyroid hormone thyroxine (T4), remains a cornerstone in treating hypothyroidism—one of the most prevalent endocrine disorders worldwide. Given its long-standing clinical use, established efficacy, and favorable safety profile, levothyroxine continues to dominate the thyroid hormone replacement market. This analysis explores current market dynamics, key growth drivers, competitive landscape, and projective sales trajectories for levothyroxine through 2030.
Market Overview
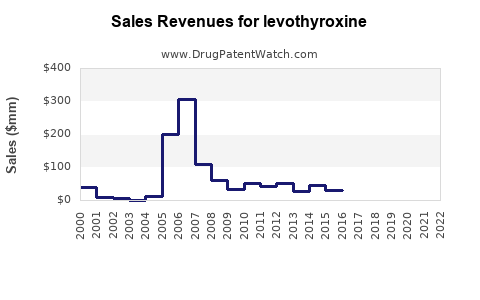
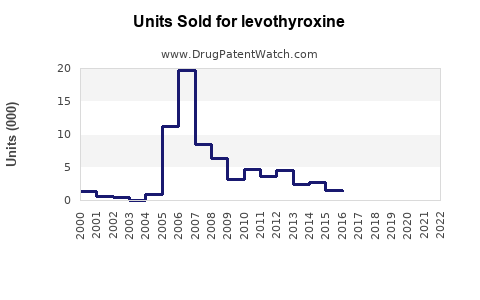
The global levothyroxine market is characterized by robust demand driven primarily by rising hypothyroidism prevalence, aging populations, and increased screening initiatives. The market is estimated to be valued at approximately USD 1.8 billion in 2022, with a compound annual growth rate (CAGR) forecast of roughly 3.5% from 2023 to 2030 [1].
Prevalence and Demographics
Hypothyroidism affects roughly 4.6% of the U.S. population aged 12 and older, with higher prevalence in women over 60 years (up to 10%) (American Thyroid Association, 2021). Growing awareness, routine screening, and the asymptomatic nature of early disease stages amplify diagnosis rates. Developing countries are witnessing an uptick in hypothyroid cases, driven by iodine deficiency, environmental factors, and improved healthcare access.
Market Drivers
-
Increased Diagnosis and Screening
Enhanced screening programs, especially in North America and Europe, have increased detection of hypothyroidism, directly fueling levothyroxine prescriptions.
-
Aging Population
Age-related decline in thyroid function necessitates ongoing hormone replacement therapy, expanding market size.
-
Brand and Generic Accessibility
The availability of generic levothyroxine (e.g., Synthroid, Euthyrox, Eltroxin) has widened access, positively impacting volumes.
-
Chronic Disease Management Trends
As hypothyroidism is a lifelong condition, steady prescription renewal sustains consistent market demand.
Market Segmentation
The product is primarily administered orally, available as tablets of varying doses. Geographic segmentation indicates North America and Europe collectively hold roughly 65% of the market share, with Asia-Pacific experiencing rapid growth potential.
Competitive Landscape
Major pharmaceutical companies, including AbbVie, Merck, and Teva, dominate the market with established manufacturing, distribution, and brand loyalty. The market is relatively consolidated, with limited room for new entrants unless novel formulations or delivery mechanisms are introduced.
Regulatory Environment
Regulatory agencies, such as the FDA and EMA, enforce stringent quality standards for thyroid hormones. Patent expirations and the availability of generics have contributed to price competition and wider access but also pose challenges for brand differentiation.
Sales Projections to 2030
Considering current trends and emerging factors, future sales projections suggest steady growth:
- 2022 Baseline: USD 1.8 billion
- 2023-2030 CAGR: 3.5%
Applying this growth rate:
| Year |
Projected Market Size (USD Billion) |
| 2023 |
~USD 1.86 |
| 2024 |
~USD 1.93 |
| 2025 |
~USD 2.00 |
| 2026 |
~USD 2.07 |
| 2027 |
~USD 2.15 |
| 2028 |
~USD 2.23 |
| 2029 |
~USD 2.30 |
| 2030 |
~USD 2.39 |
Assumptions underpinning projections:
- Continued increases in diagnosis rates and medication adherence.
- Steady or marginally increased prescription volumes driven by aging demographics.
- Incremental market penetration in emerging markets absent significant regulatory or manufacturing disruptions.
- Limited impact from patent cliffs, given dominance of generic formulations.
Potential Growth Catalysts and Risks
- Growth Catalysts: Innovation in formulations (e.g., liquid or softgel), personalized dosing, digital adherence tools, and increased focus on metabolic health.
- Risks: Market saturation in mature regions, pricing pressures from generics, regulatory hurdles, and potential shifts towards alternative therapies.
Conclusion
Levothyroxine’s market remains stable with predictable growth aligned with demographic trends. Its entrenched position, combined with increasing diagnosis rates, fosters sustained demand. Manufacturers should focus on expanding access in emerging markets, optimizing formulations, and leveraging patient adherence opportunities to reinforce market share.
Key Takeaways
- The global levothyroxine market is forecasted to reach approximately USD 2.39 billion by 2030, growing at ~3.5% annually.
- Rising hypothyroidism prevalence, especially among aging populations, remains the principal driver.
- Widespread availability of generics sustains affordability and market penetration but limits premium pricing strategies.
- Growth opportunities exist in innovative formulations and digital adherence solutions, particularly in emerging markets.
- Market stability hinges on regulatory compliance and continued clinical reliance on levothyroxine as the treatment standard.
FAQs
1. How has the COVID-19 pandemic impacted levothyroxine sales?
The pandemic caused initial disruptions in healthcare access, but overall, levothyroxine demand remained stable due to its chronic nature. Telemedicine and increased health awareness mitigated long-term impacts.
2. What are the main challenges facing the levothyroxine market?
Challenges include market saturation in developed regions, pricing pressures from generics, and potential formulation shortages or recalls that could affect patient compliance.
3. Are there emerging alternatives to levothyroxine?
While compounds like liothyronine (T3) and combination T4/T3 therapies exist, none have surpassed levothyroxine’s widespread acceptance. Research continues into personalized hormone therapy and novel delivery systems.
4. How do regulatory policies influence levothyroxine sales?
Stringent quality standards and approval processes ensure product safety but can also slow product launches or reformulations, affecting sales dynamics.
5. What strategies can manufacturers adopt to sustain growth?
Investing in formulation innovation, expanding into nascent markets, fostering direct-to-consumer education, and optimizing supply chains are key strategies.
References
- MarketWatch. “Global Levothyroxine Market Size, Trends & Forecasts (2022-2030).”