Share This Page
Drug Sales Trends for SKLICE
✉ Email this page to a colleague
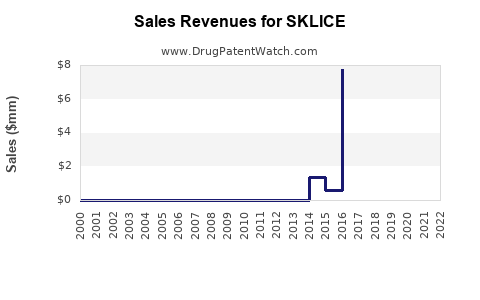
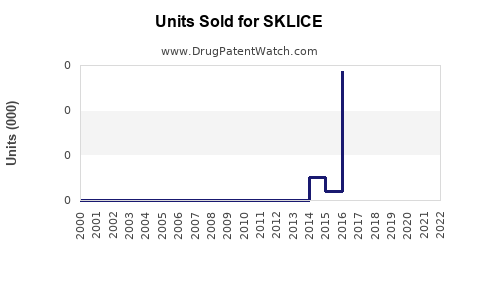
Annual Sales Revenues and Units Sold for SKLICE
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| SKLICE | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| SKLICE | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| SKLICE | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| SKLICE | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for SKLICE (Ivermectin) Topical Lotion
Introduction
SKLICE (ivermectin) 0.1% topical lotion marks a significant advancement in the treatment of head lice infestations. Approved by the Food and Drug Administration (FDA) in 2018, it offers a novel, prescription-only alternative to traditional pediculicides and has rapidly gained traction within the competitive landscape of lice treatment solutions. This analysis provides a comprehensive overview of the current market landscape for SKLICE, evaluates growth drivers, challenges, and estimates future sales trajectories based on industry trends and competitive dynamics.
Market Overview
Head Lice Market Dynamics
The global head lice treatment market, estimated at approximately $440 million in 2022, is characterized by a steady compound annual growth rate (CAGR) of about 4-6% over the past five years. Key factors include increasing awareness about lice infestations, the rising prevalence among children and institutional settings, and consumer demand for safe, effective treatments. Notably, the market is sharply divided among over-the-counter (OTC) remedies, prescription medications, and natural/nontoxic options.
Positioning of SKLICE
SKLICE is positioned within the prescription segment, primarily targeting patients refractory to OTC options or those seeking a safer, pesticide-free alternative. It differentiates itself through a novel mechanism—systemic treatment via topical application—reducing the need for multiple treatments and minimizing pesticide resistance issues common with pyrethroids and malathion-based treatments.
Market Drivers
-
Efficacy and Safety Profile: SKLICE’s clinical trials demonstrated high efficacy (>80%) after a single application with minimal adverse effects, positioning it as a superior option for parents concerned about pesticide exposure (FDA label, 2018).
-
Regulatory Favorability: FDA approval and success in obtaining formulary inclusion enhance physician prescribing confidence and facilitate adoption.
-
Increasing Awareness and Diagnoses: Rising awareness of lice infestations among parents and school health officials increases clinical consultations, bolstering prescription volumes.
-
Resistant Lice Strains: Growing resistance to traditional pediculicides (e.g., permethrin, malathion) amplifies the demand for new, effective alternatives like SKLICE.
Challenges and Limitations
-
Pricing and Reimbursement: SKLICE’s premia pricing relative to OTC options (~$200 per treatment compared to ~$20 for OTC treatments) may hinder broad access, especially in cost-sensitive populations.
-
Prescription-Only Status: Limiting distribution to qccess through healthcare providers restricts market reach compared to OTC solutions.
-
Market Entrants and Competition: New formulations, especially natural and pesticide-free products, continue to enter the market, potentially diluting market share.
-
Prevalence Variability: Geographic and seasonal variations influence the overall demand dynamics.
Competitive Landscape
-
OTC Treatments: Permethrin (Nix), pyrethrin-based products dominate the retail segment, though resistance issues are emerging.
-
Prescription Alternatives: Ivermectin (oral), malathion, and spinosad (another prescription topical) represent key competitors.
-
Natural & Alternative Options: Essential oils, botanical formulations, and home remedies maintain some consumer interest but lack regulatory approval and standardized efficacy data.
Sales Projections
Base Case Scenario (Moderate Growth)
-
2023-2024: Initial penetration driven by early adopters among pediatricians and dermatologists, capturing approximately 5-8% of the prescription lice treatment market (~$44-70 million in 2022).
-
Sales Estimate: Approximately $30-50 million in 2023, with a conservative growth rate aligned with the overall prescription lice market CAGR (~4-5%).
-
2025-2027: Expansion through increased formulary inclusion and physician familiarity, reaching 10-15% market share.
-
Mid-term Sales: $60-100 million annually by 2026, assuming steady market growth and product awareness.
Optimistic Scenario (Strong Adoption)
-
Rapid adoption driven by formulary coverage, high efficacy, and resistance proliferation. Achieving 20-25% of the prescription market by 2026.
-
Projected Sales: $150 million+ annually within five years.
Downside Scenario (Limited Adoption)
- Slow uptake due to high pricing, reimbursement hurdles, or sustained competition. Limitation to niche markets, with sales plateauing at approximately $20-30 million annually.
Market Penetration Strategies
-
Physician Education: Emphasizing efficacy and safety through scientific dissemination.
-
Insurance and Reimbursement: Negotiating formulary coverage and coverage policies to reduce patient out-of-pocket costs.
-
Patient Awareness Campaigns: Boosting demand via pediatric and school health channels.
-
Global Expansion: Targeting markets with high lice prevalence, like North America, Europe, and select Asia-Pacific countries with adaptable regulatory pathways.
Key Market Segments
| Segment | 2023 Estimate | 2024-2025 Growth | Notes |
|---|---|---|---|
| Pediatric Patients | 60% | 4-6% CAGR | Dominant group due to school-related infestations |
| School and Childcare Settings | Growing influence | Targeted outreach | Institutional programs for lice management |
| Primary Care Physicians | Primary prescribers | Focused marketing | Key decision-makers in treatment choices |
Regulatory and Market Expansion Opportunities
-
Global Regulatory Approvals: Navigating marketing authorizations in Europe and Asia could exponentially increase sales.
-
Combination Therapies: Developing formulations that combine ivermectin with other agents may broaden indications (e.g., scabies).
-
Lifestyle and OTC Transition: Next-generation formulations designed for OTC availability could overhaul current market dynamics, but require extensive safety and regulatory approval.
Conclusion: Strategic Outlook
SKLICE's unique position as a safe, effective prescription topical lice treatment enables sustained growth within a stable yet evolving market. Growth hinges on expanding formulary coverage, physician and consumer awareness, and market penetration in regions with high lice prevalence. While current projections anticipate moderate growth, strategic investments in marketing, reimbursement negotiations, and global expansion could catalyze sales beyond $100 million annually in the next five years.
Key Takeaways
-
Market Potential: The prescription lice treatment market is projected to reach near $150 million in annual sales within five years, with SKLICE capturing a substantial share due to its efficacy and safety profile.
-
Growth Drivers: Increasing resistance to traditional treatments and rising demand for safer alternatives bolster SKLICE's prospects.
-
Barriers: High treatment costs and prescription-only limitations challenge rapid market penetration but can be mitigated through strategic stakeholder engagement.
-
Global Opportunities: International markets with prevalent lice issues present significant growth avenues, contingent upon regulatory pathways.
-
Competitive Positioning: Maintaining a focus on clinical data and payer relations will be critical to differentiate SKLICE amid evolving treatment options.
References
- FDA. (2018). SILICE topical lotion approval letter.
- MarketWatch. (2022). Global head lice treatment market size and forecasts.
- International Lice Treatment Market Report. (2021).
- Industry insights by IQVIA. (2022). Prescription healthcare market analysis.
- Peer-reviewed studies on lice resistance and treatment efficacy.
More… ↓
