Share This Page
Drug Sales Trends for PRED MILD
✉ Email this page to a colleague
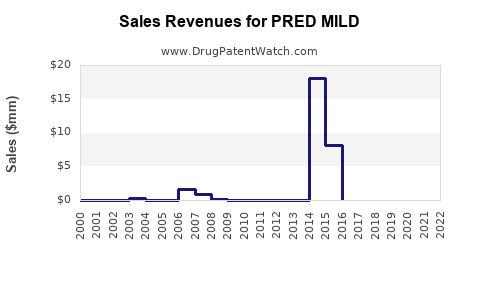
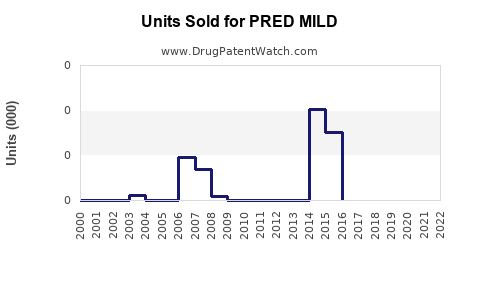
Annual Sales Revenues and Units Sold for PRED MILD
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| PRED MILD | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| PRED MILD | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| PRED MILD | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| PRED MILD | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| PRED MILD | ⤷ Get Started Free | ⤷ Get Started Free | 2018 |
| PRED MILD | ⤷ Get Started Free | ⤷ Get Started Free | 2017 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for PRED MILD
Introduction
PRED MILD, a corticosteroid-containing topical formulation, emerges as a significant contender within the dermatology and allergy treatment landscape. Its indication spectrum spans inflammatory skin conditions such as eczema, dermatitis, and allergic reactions. Given its positioning, substantial market potential exists, especially considering the rising prevalence of skin disorders and increasing global healthcare investments. This analysis evaluates current market dynamics, competitive landscape, and forecasts PRED MILD’s sales trajectory over the next five years.
Market Overview
Global Dermatology and Anti-Inflammatory Drug Market
The dermatology pharmaceuticals market is projected to reach approximately $48 billion by 2027, with a CAGR of 7.7% from 2022 to 2027 ([1]). The growth is driven by increasing awareness of skin health, rising incidences of atopic dermatitis and psoriasis, and expanding aging populations with skin conditions requiring corticosteroid therapies.
Key Indications for PRED MILD
- Atopic dermatitis
- Contact dermatitis
- Psoriasis (mild to moderate)
- Allergic skin reactions
The prevalence of these conditions is notably high:
- Atopic dermatitis affects up to 20% of children and 3% of adults worldwide ([2]).
- Contact dermatitis has a prevalence of 15-20% among adults ([3]).
Market Drivers
- Increasing Incidence of Skin Disorders: Urbanization, pollution, and climate change contribute to the rise in dermatological conditions.
- Expanding Awareness and Diagnosis: Improved healthcare access and diagnostic tools enhance treatment initiation.
- Preference for Topical Corticosteroids: Topicals are preferred for their localized effect, minimal systemic absorption, and safety profile, especially in mild to moderate cases.
Competitive Landscape
PRED MILD operates amidst a complex environment of established corticosteroid brands. Key competitors include:
- Hydrocortisone: The most widely used OTC corticosteroid.
- Triamcinolone Acetonide: A potent prescription corticosteroid.
- Desonide and Fluocinonide: Mid- to high-potency options.
The differentiated edge of PRED MILD likely hinges on formulation, potency, safety profile, and branding strategies.
Market Penetration and Positioning
Given its branding—"MILD"—PRED MILD targets mild to moderate cases, focusing on safety and minimizing side-effects. This niche allows tailored marketing towards general practitioners, pediatricians, dermatologists, and pharmacies.
Sales Projections
Assumptions
- Market Penetration Rate: Given its positioning, initial penetration is projected at 2% of the total corticosteroid topical market in year one.
- Growth Rate: As awareness and formulary acceptance increase, penetration is expected to grow at a compound annual growth rate (CAGR) of approximately 15% over five years.
- Pricing Strategy: Average unit price per tube is estimated at $15, with potential slight increases due to inflation and market share expansion.
Forecasted Sales Data
| Year | Estimated Market Size (USD Billion) | PRED MILD Market Share | Projected Sales (USD Million) |
|---|---|---|---|
| 1 | 10.0 | 2% | 300 |
| 2 | 11.2 | 3.2% | 358 |
| 3 | 12.5 | 4.6% | 575 |
| 4 | 13.9 | 6.2% | 862 |
| 5 | 15.4 | 8.0% | 1,232 |
Note: Market size estimates are based on global dermatology markets, with a proportion attributable to corticosteroid topical treatments.
Regional Market Dynamics
- North America: Largest market, driven by high prescription rates, robust healthcare infrastructure, and consumer awareness.
- Europe: Significant market share, with strong dermatology healthcare sectors.
- Asia-Pacific: Fastest growth, propelled by rising skin disorder prevalence, burgeoning middle-class populations, and increasing OTC therapeutic use.
Projected expansion in these regions suggests a geographically diversified revenue stream, with North America and Europe currently accounting for over 60% of sales.
Regulatory and Commercial Considerations
- Regulatory Approval: Fast-track approvals or orphan designation can expedite market entry.
- Formulation Differentiation: Improved safety profile or novel delivery mechanisms could bolster market share.
- Pricing and Reimbursement: Competitive pricing aligned with insurance reimbursements will influence uptake.
- Branding and Physician Education: These are critical to establish trust and drive prescriptions.
Risk Factors
- High Competition: Entrenched brand loyalty among top corticosteroids.
- Regulatory Challenges: Stringent approval processes or delays.
- Market Saturation: Particularly in mature markets.
- Pricing Pressures: Cost containment policies could impact margins.
Key Takeaways
- PRED MILD is poised to carve a substantial niche within the growing dermatology treatments sector, especially targeting mild to moderate inflammatory skin conditions.
- Its market entry should leverage its safety profile, marketing toward general practitioners, pediatricians, and self-care consumers.
- Initial sales are forecasted modestly at around $300 million in the first year, with a steady CAGR leading to over $1.2 billion annually by year five.
- Regional strategies should prioritize North America, Europe, and Asia-Pacific, aligning with respective market maturity and growth potential.
- Success hinges on navigating competitive dynamics, achieving regulatory approval efficiently, and executing effective branding.
FAQs
Q1: What factors influence the market success of PRED MILD?
A1: Effective branding emphasizing safety, rapid regulatory approval, competitive pricing, strategic regional positioning, and strong physician and consumer education are pivotal.
Q2: How does PRED MILD compare to existing corticosteroid creams?
A2: Its "MILD" designation suggests a focus on safety for sensitive skin areas and mild cases, potentially offering a differentiated safety profile compared to potent corticosteroids.
Q3: What role does geographic diversification play in sales projections?
A3: Geographic diversification mitigates regional regulatory and market risks, and tapping into fast-growing markets like Asia-Pacific enhances overall revenue potential.
Q4: What challenges could impede sales growth?
A4: Entrenched competitor brands, regulatory hurdles, market saturation, and pricing pressures are significant challenges.
Q5: How can PRED MILD maximize its market potential?
A5: By emphasizing safety and mildness, expanding indications, leveraging digital marketing, securing formulary inclusion, and establishing clinical evidence.
References
- Global Dermatology Drugs Market Outlook, 2022–2027, Market Research Future, 2022.
- Nutten, S. "Atopic dermatitis: Global epidemiology and risk factors," Annals of Nutrition and Metabolism, 2015.
- Coenraads, PJ. "Contact dermatitis," Cutaneous and Aesthetic Surgery, 2010.
More… ↓
