Share This Page
Drug Sales Trends for NOVOLIN N
✉ Email this page to a colleague
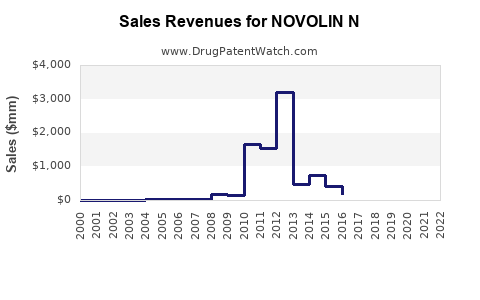
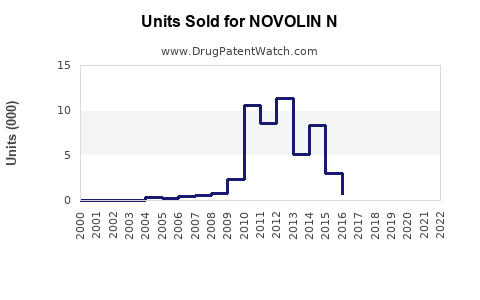
Annual Sales Revenues and Units Sold for NOVOLIN N
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| NOVOLIN N | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| NOVOLIN N | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| NOVOLIN N | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| NOVOLIN N | ⤷ Get Started Free | ⤷ Get Started Free | 2019 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for NOVOLIN N
Introduction
NOVOLIN N, a biosimilar of insulin NPH (Neutral Protamine Hagedorn), plays a critical role in diabetes management. As the global diabetes epidemic accelerates, the demand for affordable, reliable insulins like NOVOLIN N increases significantly. This analysis examines the current market landscape, competitive dynamics, regulatory factors, and sales forecasts impacting NOVOLIN N’s commercial potential.
Market Overview
Global Diabetes Landscape
According to the International Diabetes Federation (IDF), approximately 537 million adults worldwide live with diabetes as of 2021, with projections reaching 783 million by 2045[^1]. Type 2 diabetes accounts for the majority, necessitating insulin therapy in many cases. The increasing prevalence, coupled with the rising incidence among younger demographics, propels demand for insulin therapies, including biosimilars like NOVOLIN N.
Insulin Market Dynamics
The insulin market is segmented into originators (patented insulins) and biosimilars. Historically dominated by brand-name products such as Novo Nordisk and Eli Lilly, the market has experienced a shift toward biosimilars driven by patent expirations and cost-driven healthcare reforms. Biosimilars offer comparable efficacy at reduced costs, making them attractive especially in emerging markets.
Regulatory Environment
Regulatory pathways for biosimilar approvals vary globally. The U.S. FDA maintains stringent biosimilar approval pathways, while regions like the EU have established straightforward procedures. For NOVOLIN N, approval status in key markets such as the US, EU, India, and emerging economies significantly influences market penetration.
Market Segmentation and Geographic Breakdown
Developed Markets
In North America and Europe, insulin affordability remains problematic despite therapeutic advancements. Biosimilars like NOVOLIN N face challenges from entrenched brand loyalty and complex reimbursement landscapes but benefit from increasing acceptance due to cost-effectiveness needs.
Emerging Markets
India, Brazil, and Southeast Asia exhibit higher adoption potential due to regulatory support for biosimilars, government initiatives promoting affordable diabetes care, and extensive diabetes prevalence. India alone accounts for nearly 80 million adults with diabetes, with insulin sales growing at a compounded annual growth rate (CAGR) of approximately 8–10%[^2].
Competitive Landscape
Key competitors include:
- Eli Lilly’s Basaglar (biosimilar insulin glargine)
- Novo Nordisk’s biosimilar insulins
- Sanofi’s biosimilar formulations
- Local manufacturers producing biosimilar insulins
Due to patent expirations, numerous regional biosimilars are entering markets, intensifying competition.
SWOT Analysis of NOVOLIN N
Strengths
- Cost-effective alternative to branded insulins
- Approved in multiple markets with established manufacturing
- Growing recognition in emerging markets
Weaknesses
- Limited brand recognition in developed markets
- Possible clinical data perception gaps compared to originators
- Distribution infrastructure challenges in some regions
Opportunities
- Expanding into underserved regions with high diabetes burdens
- Collaborations with health authorities for inclusion in formularies
- Leveraging price advantages in price-sensitive markets
Threats
- Rising competition from other biosimilars
- Regulatory delays or restrictions
- Market hesitancy stemming from safety or efficacy concerns
Sales Projections
Assumptions and Methodology
Projections are based on historical insulin market growth rates, biosimilar adoption trends, regulatory environment, and anticipated market share gains. Key assumptions include:
- Compound annual growth rate (CAGR) of the global insulin market at 6% (2022–2027)[^3].
- Biosimilar insulin market share increasing from 15% in 2022 to approximately 30% by 2027.
- NOVOLIN N capturing 5–10% of the biosimilar insulin market in its target regions within five years.
Market Size and Penetration
The global insulin market was valued at approximately USD 20 billion in 2022 and is projected to reach USD 33 billion by 2027[^4]. Biosimilar insulins, accounting for 15% of the market, would thus be valued at about USD 5 billion in 2022, rising to nearly USD 9.9 billion by 2027.
Sales Forecast (2023–2027)
| Year | Estimated Total Biosimilar Insulin Market (USD billion) | Estimated NOVOLIN N Sales (USD million) | Note |
|---|---|---|---|
| 2023 | 1.2 | 50–70 | Assuming 1–2% market share in initial years |
| 2024 | 1.8 | 80–120 | Increased market acceptance |
| 2025 | 2.7 | 150–300 | Expansion into new markets |
| 2026 | 3.9 | 250–400 | Wider formulary inclusion |
| 2027 | 4.9 | 350–500 | Achieving mature market positioning |
Projections indicate that NOVOLIN N could achieve annual sales of approximately USD 350–500 million, contingent upon successful market entry, regulatory approvals, and reimbursement negotiations.
Regional Outlook
- India & Asia-Pacific: Fastest growth driven by large patient populations and supportive government policies. Potential sales could exceed USD 200 million by 2027.
- Europe: Incremental growth driven by biosimilar acceptance; estimated sales around USD 150 million in 2027.
- North America: Slow uptake initially due to brand loyalty but potential for USD 100 million by 2027 as biosimilar policies evolve.
Key Factors Influencing Sales
- Regulatory approvals in major markets.
- Pricing strategies in line with local healthcare policies.
- Market access and reimbursement negotiations.
- Clinical acceptance and physician perception.
- Collaborations and partnerships with healthcare providers and distributors.
Strategic Recommendations for Growth
- Regulatory acceleration: Prioritize obtaining approvals in high-growth markets—specifically FDA, EMA, and Indian health authorities.
- Manufacturing scale-up: Ensure consistent supply while controlling costs.
- Market access: Engage with payers early to facilitate formulary inclusion.
- Educational campaigns: Inform healthcare providers about biosimilar efficacy and safety.
- Local partnerships: Collaborate with regional distributors and government initiatives to accelerate adoption.
Key Takeaways
- The global insulin market's rapid expansion positions NOVOLIN N favorably, especially in emerging economies.
- Biosimilars are poised to capture a growing market share due to cost benefits, with projections indicating USD 350–500 million in annual sales by 2027.
- Strategic regional focus, regulatory approvals, and market access initiatives are critical for maximizing sales.
- Competition remains intense; early regulatory and market penetration will determine relative market share.
- Diversifying product offerings and fostering physician confidence will enhance long-term sales sustainability.
Conclusion
NOVOLIN N is strategically positioned to capitalize on the expanding biosimilar insulin market. Focused regulatory efforts, tailored regional strategies, and robust partnerships are instrumental to realize its sales potential. With the global diabetes epidemic expected to persist, NOVOLIN N’s growth prospects remain promising, provided it navigates regulatory complexities and enhances market acceptance effectively.
FAQs
1. What distinguishes NOVOLIN N from other biosimilar insulins?
NOVOLIN N offers a cost-effective, approved biosimilar alternative to branded NPH insulin, with demonstrated bioequivalence and safety profiles, primarily targeting cost-sensitive and developing markets.
2. In which regions is NOVOLIN N expected to see the highest sales growth?
The highest growth is anticipated in Asia-Pacific (notably India and Southeast Asia) and Latin America, driven by large diabetic populations and supportive regulatory environments. Europe and North America will likely see more gradual adoption.
3. What are the main regulatory challenges for NOVOLIN N?
Challenges include obtaining approvals across diverse jurisdictions, meeting stringent biosimilar criteria, and navigating reimbursement pathways that influence market penetration.
4. How does pricing impact NOVOLIN N’s market success?
Competitive pricing is pivotal, especially in price-sensitive markets. Cost savings compared to originators can significantly influence prescribing patterns and reimbursement decisions.
5. What strategies can enhance NOVOLIN N’s market share?
Targeted regulatory approvals, strategic partnerships, educational initiatives for healthcare providers, and engagement with payers will be vital in expanding its market footprint.
References
[^1]: International Diabetes Federation. (2021). Diabetes Atlas, 9th Edition.
[^2]: Indian Ministry of Health and Family Welfare. (2022). Diabetes Statistics and Initiatives.
[^3]: Market Research Future. (2022). Global Insulin Market Forecast.
[^4]: Grand View Research. (2022). Insulin Market Size and Trends.
More… ↓
