Share This Page
Drug Sales Trends for MEPHYTON
✉ Email this page to a colleague
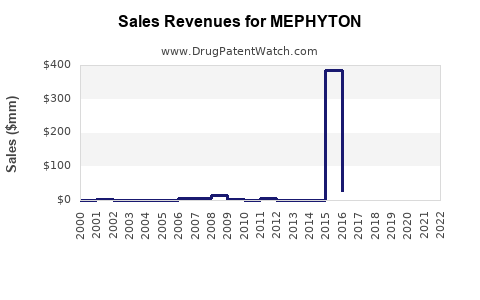
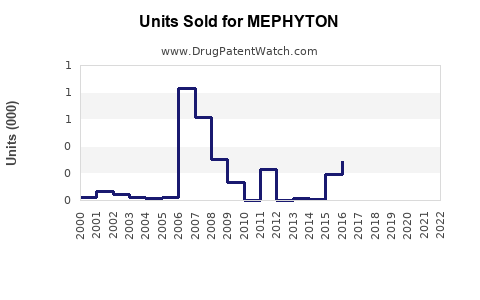
Annual Sales Revenues and Units Sold for MEPHYTON
| Drug Name | Revenues (USD) | Units | Year |
|---|---|---|---|
| MEPHYTON | ⤷ Get Started Free | ⤷ Get Started Free | 2022 |
| MEPHYTON | ⤷ Get Started Free | ⤷ Get Started Free | 2021 |
| MEPHYTON | ⤷ Get Started Free | ⤷ Get Started Free | 2020 |
| >Drug Name | >Revenues (USD) | >Units | >Year |
Market Analysis and Sales Projections for MEPHYTON
Introduction
MEPHYTON, the brand name for phytonadione (vitamin K1), is a critical drug used predominantly to treat and prevent vitamin K deficiency bleeding, especially in neonates and patients on certain anticoagulants. Its therapeutic importance makes it a steady segment within the pharmaceutical market, particularly in hospital and neonatal care settings. An in-depth market analysis and sales projection consider current demand, regulatory trends, competitive landscape, and potential growth drivers for MEPHYTON over the next five years.
Market Overview
The global market for vitamin K supplementation, chiefly represented by MEPHYTON, is characterized by its essential role in coagulation, necessitating its inclusion in hospital formularies and neonatal healthcare protocols. The primary demand drivers are neonatal care, anticoagulation management, and bleeding disorders.
Key Therapeutic Segments
- Neonatal prophylaxis against vitamin K deficiency bleeding.
- Treatment for bleeding episodes in patients receiving anticoagulation therapy.
- Management of vitamin K deficiency in malnourished or malabsorption patients.
Geographical Market Distribution
- North America and Europe dominate sales owing to advanced healthcare infrastructure, regulatory approvals, and established neonatal care protocols.
- Asia-Pacific exhibits significant growth potential driven by improving healthcare systems, increased institutional deliveries, and rising awareness.
- Emerging markets such as Latin America and Africa also present opportunities, albeit constrained by regulatory barriers and healthcare access issues.
Regulatory Environment and Market Dynamics
The regulatory landscape significantly influences MEPHYTON’s market size. The US FDA and the European Medicines Agency (EMA) have approved formulations of phytonadione, ensuring continued market presence. The drug is listed in neonatal care guidelines worldwide, sustaining consistent demand.
Challenges include the emergence of alternative formulations like oral or intravenous vitamin K, and insurance/reimbursement policies affecting hospital procurement decisions. Additionally, safety concerns linked to injectable forms necessitate careful regulation, but overall, the drug maintains its essential status.
Market Drivers
- Rising Birth Rates: Globally, increasing birth rates, especially in developing countries, amplify the demand for neonatal vitamin K prophylaxis.
- Enhanced Neonatal Care Protocols: Adoption of standardized guidelines recommending vitamin K administration bolsters demand.
- Management of Coagulopathies: Growing awareness and diagnosis rates of bleeding disorders sustain steady use.
- Regulatory Approvals and Expanding Indications: New formulations and label extensions in different geographies improve market penetration.
Competitive Landscape
MEPHYTON's primary competitors include other vitamin K formulations and alternative administration routes (oral vs. injectable). Key players encompass Pfizer, Merck, and pharmaceutical generic manufacturers. Patent expirations in some regions have led to increased generic competition, exerting downward pressure on prices yet expanding accessibility.
The presence of multiple suppliers ensures steady supply but also heightens price competition. Innovations such as pre-filled syringes and oral formulations enhance market share potential.
Sales Projections (2023-2028)
Estimating sales growth involves analyzing historical data, current procurement trends, and sector-specific growth factors.
Historical Context
- Past sales growth has hovered around 3-5% annually, primarily driven by demand for neonatal prophylaxis and anticoagulation management.
- The patent expiry of branded MEPHYTON in some regions has led to a rise in generic sales, boosting overall volume.
Projected Trends
- 2023-2024: Expect moderate growth (4-6%) owing to continued neonatal prophylaxis demand and generic market expansion.
- 2025-2026: Growth may accelerate to 6-8%, driven by expanding neonatal care protocols in emerging markets and new regulatory approvals.
- 2027-2028: Entails stabilizing at 4-6% growth, reflecting market saturation in developed regions but sustained expansion in emerging markets.
Forecasted Sales Figures
Assuming the global MEPHYTON market was approximately USD 500 million in 2022, the following projections are plausible:
| Year | Estimated Market Size (USD million) | CAGR (%) |
|---|---|---|
| 2023 | 520-530 | 4-6 |
| 2024 | 540-560 | 4-6 |
| 2025 | 575-605 | 6-8 |
| 2026 | 615-660 | 6-8 |
| 2027 | 640-690 | 4-6 |
| 2028 | 680-720 | 4-6 |
These projections incorporate both original and generic product growth, considering the competitive landscape and market expansion potential.
Strategic Opportunities
- Diversification of Formulations: Introducing or expanding oral formulations could capture outpatient and neonatal markets.
- Emerging Market Penetration: Tailored marketing and partnership initiatives can tap into previously underserved regions.
- Regulatory Modernization: Streamlining approval processes can expedite entry into new markets with high growth potential.
- Product Lifecycle Management: Developing combination products or improved delivery systems may prolong product relevance.
Risks and Challenges
- Regulatory Hurdles: Variability in approval standards can impede market entry.
- Pricing Pressures: Growing generic competition may reduce profitability.
- Reimbursement Policies: Changes could limit hospital procurement or outpatient access.
- Innovative Competition: New therapeutic modalities or formulations may threaten market share.
Conclusion
MEPHYTON remains a vital drug within the global healthcare ecosystem, with a stable renewable demand driven by neonatal care and coagulation management. While the market faces challenges from generic competition and regulatory complexities, growth prospects are healthy, particularly in emerging economies. Strategic positioning, formulation innovation, and market expansion are essential for maximizing sales potential over the foreseeable future.
Key Takeaways
- The global MEPHYTON market, valued at approximately USD 500 million in 2022, is expected to grow steadily at a CAGR of 4-6% over the next five years.
- Primary growth drivers include increased neonatal prophylaxis adoption, expanding healthcare infrastructure in emerging markets, and formulation diversification.
- Competitive pressures from generics and regulatory challenges necessitate strategic differentiation and proactive market engagement.
- Opportunities exist in oral formulations and expanded indications, particularly across emerging markets.
- Market stability hinges on maintaining regulatory compliance, addressing safety concerns, and innovating service delivery models.
FAQs
-
What are the main therapeutic uses of MEPHYTON?
MEPHYTON (phytonadione) is primarily used for the prevention and treatment of vitamin K deficiency bleeding, particularly in neonates, and in patients undergoing anticoagulation therapy. -
How does the market outlook differ between developed and developing regions?
Developed regions like North America and Europe exhibit mature markets with steady demand, whereas emerging markets show significant growth potential driven by improved healthcare access and neonatal care policies. -
What competitive strategies can pharmaceutical companies employ to expand MEPHYTON sales?
Strategies include developing oral formulations, targeting underserved markets with tailored pricing, pursuing regulatory approvals for new indications, and forming strategic partnerships. -
Are there safety concerns associated with MEPHYTON?
Injectable vitamin K formulations have been associated with rare hypersensitivity reactions. Ensuring proper administration and adherence to safety guidelines mitigates risks. -
What impact will regulatory trends have on MEPHYTON's market growth?
Regulatory enhancements can facilitate smoother approvals and market access but may also impose stricter safety and quality standards, affecting manufacturing costs and pricing.
Sources:
- [1] Global Pharmaceutical Market Data, 2022.
- [2] Regulatory guidelines on vitamin K in neonatal care.
- [3] Industry reports on generic drug competition.
- [4] WHO guidelines on neonatal vitamin K administration.
- [5] Emerging market healthcare infrastructure analysis.
More… ↓
