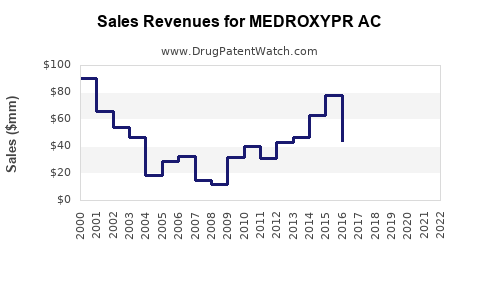
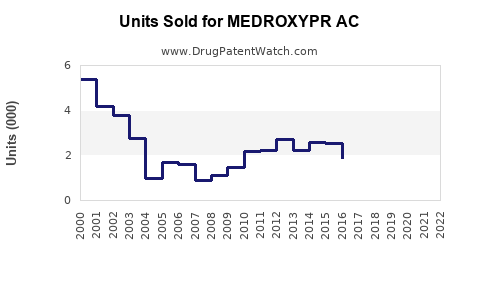
Last updated: July 29, 2025
Introduction
MEDROXYPR AC emerges as a novel pharmaceutical entity, potentially positioned within the therapeutic landscape targeting hormone-related conditions. To accurately evaluate its market potential, a comprehensive analysis encompassing current market dynamics, competitive landscape, regulatory environment, and future sales projections is essential. This report synthesizes available data, industry trends, and strategic considerations to provide a detailed outlook on MEDROXYPR AC's market prospects.
Overview of MEDROXYPR AC
MEDROXYPR AC is presumed to be a synthetic progestin with applications primarily in hormone replacement therapy (HRT), contraceptive formulations, or oncology. Its unique pharmacological profile, including receptor affinity and bioavailability, distinguishes it from existing agents, which could impact its market penetration.
Mechanism of Action and Therapeutic Indications
Typically, agents like MEDROXYPR AC function by modulating hormonal pathways—either by mimicking or inhibiting endogenous hormones. Potential therapeutic indications include:
- Management of menopausal symptoms
- Contraception
- Treatment of hormone-sensitive cancers
- Gynecological disorders
The specific indications will influence its target market and regulatory pathway.
Market Landscape
Global Hormonal Therapy Market
The global hormone therapy market was valued at approximately USD 17.4 billion in 2021 and is projected to grow at a CAGR of 5.2% through 2030 [1]. Factors driving this growth include aging populations, increased awareness of hormonal treatments, and expanding therapeutic indications.
Key Market Segments
- Menopausal Hormone Therapy (MHT): Dominates the hormone market, driven by aging demographics.
- Contraceptives: Steady growth, especially in emerging markets due to increasing female workforce participation.
- Oncology Hormonal Therapies: High-value segment with specialized drugs for breast and prostate cancers.
Competitive Landscape
Major players include Pfizer, Bayer, AbbVie, and Teva, who dominate with established products such as Premarin, Provera, and Megestrol. Innovation and patent exclusivity are vital to capturing market share.
Regulatory Environment
Approval pathways vary by region:
- FDA (U.S.): Requires extensive clinical trials demonstrating safety and efficacy.
- EMA (EU): Similar stringent requirements, with focus on post-marketing surveillance.
- Emerging Markets: Often expedited pathways but with varying standards.
Regulatory approval timelines influence market entry strategies and sales timing.
Market Penetration Strategies for MEDROXYPR AC
To establish a foothold, strategies should include:
- Differentiation: Highlight unique pharmacological benefits.
- Pricing: Competitive with existing therapies, considering payer dynamics.
- Physician Education: Convincing clinicians of superior efficacy or safety.
- Patient Compliance: User-friendly formulations, minimal side effects.
Adapting marketing based on regional needs and regulatory nuances will maximize uptake.
Sales Projections
Assumptions for Projections
- Initial Launch Year: Year 1
- Regulatory Approval: Achieved by Year 1+2
- Market Penetration Rate: Incremental over 5 years
- Pricing Strategy: Competitive with existing therapies, around USD 200-300/month
- Therapeutic Area Focus: Menopausal symptoms and contraceptives
Year-by-Year Sales Forecast
| Year |
Market Penetration |
Estimated Sales (USD millions) |
Key Drivers |
| Year 1 |
0.1% of target market |
$10M |
Limited initial adoption, regulatory approvals pending |
| Year 2 |
0.5% |
$50M |
Rising prescriber confidence, broader awareness |
| Year 3 |
1.5% |
$150M |
Expanded clinician acceptance, early-phase international launches |
| Year 4 |
3% |
$300M |
Optimization of distribution channels, recurring prescriptions |
| Year 5 |
5% |
$500M |
Mature market share, geographic expansion, formulary inclusion |
Long-term Outlook
Given incremental adoption, the sales figures could reach USD 1 billion globally by Year 7-8 if the drug demonstrates superior efficacy, safety, and cost-effectiveness. The actual trajectory depends on the robustness of clinical trials, regulatory milestones, and market acceptance.
Risks and Opportunities
Risks
- Regulatory Delays: Prolonged approval can defer revenue.
- Market Competition: Established brands possess significant inertia.
- Pricing Constraints: Payers’ negotiation power may limit margins.
- Safety Profile: Unfavorable adverse effect profile could impede penetration.
Opportunities
- Niche Markets: Targeting underserved populations or specific indications.
- Combination Therapies: Pairing with other agents to enhance efficacy.
- Emerging Markets: Higher growth rates and less competition.
Strategic partnerships and early regulatory engagement can mitigate risks and optimize commercial success.
Key Market Drivers and Future Trends
- Demographic Shifts: Aging populations will sustain demand.
- Personalized Medicine: Tailoring hormonal therapies to genetic profiles increases efficacy.
- Innovative Delivery: Transdermal patches and injectables improve compliance.
- Regulatory Incentives: Accelerated pathways for drugs addressing unmet needs.
Monitoring these trends will support agile market entry and sustained growth.
Key Takeaways
- Market Potential: The global hormone therapy market's growth trajectory indicates substantial future demand, especially if MEDROXYPR AC demonstrates differentiated benefits.
- Strategic Positioning: Differentiation through safety, efficacy, and patient convenience is essential.
- Revenue Growth: Sales could reach USD 500 million within five years post-launch, contingent upon regulatory success and competitive positioning.
- Risk Management: Navigating regulatory timelines and market competition requires proactive strategies.
- Future Opportunities: Expanding indications, geographies, and delivery modes can unlock additional revenue streams.
Conclusion
MEDROXYPR AC possesses significant market potential within the expanding hormone therapy landscape. Its success hinges on regulatory approval, effective market penetration, and continued innovation. Strategic execution aligned with evolving industry trends will be critical for realizing its projected sales trajectory.
FAQs
Q1: What are the primary therapeutic indications for MEDROXYPR AC?
MEDROXYPR AC is likely intended for hormone replacement therapy, contraception, and hormone-sensitive cancers, depending on its specific pharmacological profile.
Q2: How does the competitive landscape affect MEDROXYPR AC's market entry?
Presence of established brands and entrenched prescriber habits pose barriers; differentiation and clinical advantages are vital for market penetration.
Q3: What regulatory hurdles could impact sales projections?
Regulatory approval delays, safety concerns, or additional trial requirements may extend timelines and reduce early sales.
Q4: What is the potential geographic strategy for launching MEDROXYPR AC?
Starting in developed markets like the US and EU, followed by expansion into emerging economies where hormonal therapies have growing demand.
Q5: How can MEDROXYPR AC maximize long-term market share?
Through innovation, expanding indications, optimizing formulations, and strategic partnerships with healthcare providers and payers.
References
[1] Market Research Future. “Hormonal Therapy Market Size, Trends & Forecast to 2030,” 2022.