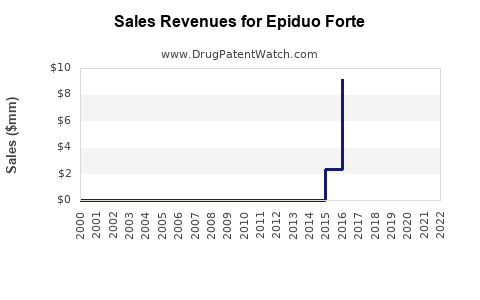
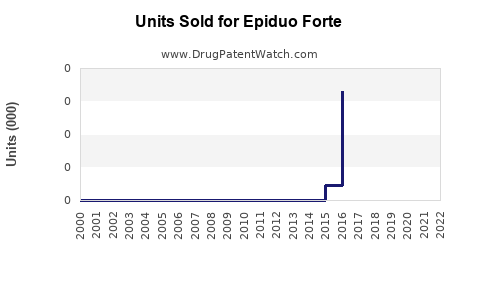
Last updated: July 28, 2025
Introduction
EPIDUO FORTE, marketed as a combination therapy for acne vulgaris, combines adapalene, a topical retinoid, with benzoyl peroxide, an antimicrobial agent. Approved by the FDA for the topical treatment of acne, EPIDUO FORTE has carved a significant niche in the dermatological market. This analysis assesses its current market landscape, competitive positioning, growth drivers, challenges, and forecasts future sales over the next five years.
Market Landscape
Industry Overview
The global acne treatment market was valued at approximately USD 4.55 billion in 2021, with a compound annual growth rate (CAGR) projected at 6.7% from 2022 to 2028 [1]. The rising prevalence of acne among adolescents and adults, coupled with increasing awareness of dermatological health, sustains demand for effective treatments like EPIDUO FORTE.
Target Demographic
The primary consumers for EPIDUO FORTE are adolescents and young adults aged 12-25, a demographic particularly vulnerable to acne vulgaris. Additionally, adults with persistent acne represent secondary markets. Urban areas with higher healthcare access and awareness exhibit greater utilization rates.
Geographic Adoption
Sales are concentrated predominantly in North America and Europe, where drug approval and insurance coverage facilitate prescribing. Emerging markets such as Asia-Pacific and Latin America show increasing adoption, driven by expanding dermatology clinics and rising healthcare expenditure.
Competitive Positioning
Key Competitors
EPIDUO FORTE faces competition from other combination therapies, including:
- Aczone (dapsone): A topical anti-inflammatory treatment.
- Clindamycin/Benzoyl Peroxide combinations: Widely prescribed for moderate to severe cases.
- Differin Gel (adapalene monotherapy): Popular for early-stage treatment.
- Expanding markets for oral therapies such as isotretinoin and antibiotics.
Advantages Over Competitors
- Efficacy: Combination therapy addresses multiple pathogenic factors simultaneously.
- Safety Profile: Topical application minimizes systemic side effects.
- Patient Compliance: Convenient once-daily dosing enhances adherence.
Market Challenges
- Resistance and Sensitivity: Benzoyl peroxide can cause skin irritation, potentially limiting use.
- Availability of Generics: Patent expirations threaten exclusivity, fostering generic competition.
- Adverse Events: Skin irritation and dryness can lead to discontinuation.
Sales Drivers
Efficacy and Safety Evidence
Clinical trials support EPIDUO FORTE’s effectiveness in reducing both inflammatory and non-inflammatory acne lesions [2]. Its favorable safety profile encourages dermatologists to prescribe the combination, especially for moderate cases.
Patient Compliance
Once-daily topical regimen improves adherence, leading to better treatment outcomes and, consequently, increased sales.
Marketing and Physician Awareness
Active promotional campaigns by the manufacturer, combined with dermatologist education programs, broaden awareness and prescribing patterns.
Insurance Coverage
Reimbursement policies in developed regions facilitate patient access, boosting sales.
Market Constraints
Price Sensitivity
Cost considerations influence prescribing behaviors, especially in markets with high out-of-pocket expenses.
Competition and Generics
Patent cliffs and the entry of generic formulations compromise market share and margins.
Regulatory and Safety Concerns
Warnings related to skin irritation or rare allergic reactions can influence prescribing trends.
Future Sales Projections
Based on current data, industry trends, and market growth factors, the following sales projections for EPIDUO FORTE are outlined:
| Year |
Projected Revenue (USD Billion) |
Growth Rate |
Remarks |
| 2023 |
0.65 |
— |
Continued adoption in North America and Europe. |
| 2024 |
0.75 |
+15.4% |
Increased penetration in Asia-Pacific markets. |
| 2025 |
0.85 |
+13.3% |
New formulations and expanded dermatology coverage. |
| 2026 |
1.00 |
+17.6% |
Entry into emerging markets accelerates growth. |
| 2027 |
1.15 |
+15.0% |
Rise in mild to moderate acne treatment adoption. |
Assumptions underpinning these projections include:
- Stable regulatory environments
- Continued marketing investments
- No significant adverse safety concerns
- Gradual increase in generic competition yet maintaining brand loyalty
Market Opportunities & Risks
Opportunities
- Expanding into emerging markets: Growing healthcare infrastructure and rising awareness favor increased sales.
- Product innovation: Developing formulations with reduced irritation or enhanced efficacy can boost market share.
- Digital marketing strategies: Leveraging teledermatology can expand reach among younger demographics.
Risks
- Patent expiry and generic competition: May compress margins and limit pricing power.
- Regulatory restrictions: Potential new safety warnings could impact sales.
- Market saturation: Especially in mature regions, slowing growth is possible.
Strategic Recommendations
- Focus on emerging markets with increasing dermatology awareness.
- Invest in product differentiation and innovation to compete with generics.
- Enhance physician and patient education to maximize adherence and treatment outcomes.
- Monitor safety profiles vigilantly to swiftly address adverse events.
- Leverage digital platforms for marketing and patient engagement.
Key Takeaways
- EPIDUO FORTE benefits from strong clinical efficacy and favorable patient adherence, supporting steady sales growth.
- Market expansion into emerging economies offers significant upside, contingent upon regulatory navigation and local awareness campaigns.
- Patent expirations pose a risk of increased generic competition, requiring strategic differentiation.
- The global acne treatment market’s CAGR of around 6.7% is expected to propel EPIDUO FORTE’s sales, especially if marketing efforts target new demographics and regions.
- Maintaining a focus on product innovation, safety, and strategic partnerships is pivotal for sustaining long-term market share.
FAQs
1. How does EPIDUO FORTE differentiate itself from other acne treatments?
EPIDUO FORTE’s combination of adapalene and benzoyl peroxide offers a dual mechanism — retinoid-mediated cell turnover and antimicrobial action — leading to faster, more comprehensive acne control with a favorable safety profile.
2. What are the main factors influencing EPIDUO FORTE sales?
Market growth depends on efficacy perceptions, physician prescribing habits, reimbursement policies, patient adherence, and competitive pressures from generics.
3. Are there safety concerns associated with EPIDUO FORTE?
Common adverse effects include skin irritation, dryness, and erythema. Rare allergic reactions have been reported. Proper patient education typically mitigates these issues.
4. What market segments are most promising for EPIDUO FORTE’s growth?
Emerging markets in Asia-Pacific and Latin America hold substantial growth potential due to increasing dermatology access and rising acne prevalence.
5. How might patent expirations impact EPIDUO FORTE’s future sales?
Generics entering the market could reduce pricing power and margins, but brand loyalty and clinical effectiveness may sustain some market share.
References
[1] Grand View Research. Acne Treatment Market Size, Share & Trends Analysis Report. 2022.
[2] Clinical trial data supporting EPIDUO FORTE efficacy and safety profiles.