Last updated: July 29, 2025
Introduction
ARMOUR THYRO (brand name: Armour Thyroid) is a well-established thyroid hormone replacement therapy primarily indicated for hypothyroidism treatment. With a longstanding presence in the endocrine therapeutics domain, Armour Thyroid's market performance hinges on factors such as clinical demand, competitive landscape, regulatory developments, and emerging trends in personalized medicine. This analysis examines current market dynamics, forecasts future sales, and offers strategic insights pertinent to stakeholders considering investments or market positioning.
Market Overview
Product Profile and Medical Context
Armour Thyroid is a desiccated thyroid extract derived from porcine thyroid glands, comprising both thyroxine (T4) and triiodothyronine (T3). It serves as a biologically active thyroid hormone replacement, favored by certain clinicians and patients seeking natural or less synthetic alternatives. Its use is anchored in treating hypothyroidism—a condition affecting an estimated 5% of women and 0.5% of men globally, predominantly in the elderly population ([1]).
Market Size and Trends
The global market for thyroid hormone replacement therapy was valued at approximately USD 1.4 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 4-5% through 2030 ([2]). Factors fueling growth include rising hypothyroidism prevalence, increased awareness, and a trend toward personalized treatment regimens. Notably, the market share between synthetic levothyroxine (L-T4) and natural desiccated thyroid products like Armour varies geographically, with the latter maintaining a niche primarily in North America and Europe.
Key Competitors
Primary competitors include synthetic levothyroxine formulations (e.g., Synthroid, Euthyrox), liothyronine (synthetic T3), and other natural desiccated thyroid products (e.g., Nature-Throid, WP Thyroid). Synthetic options dominate due to standardized dosing, regulatory approvals, and broad physician acceptance, but Armour’s unique appeal lies in its natural composition and historical brand recognition.
Regulatory Environment
FDA Stance and Regulatory Changes
In the U.S., Armour Thyroid remains an FDA-approved prescription medication. However, concerns related to batch-to-batch consistency, standardization of hormone content, and manufacturing practices have prompted regulatory scrutiny. The FDA has emphasized the importance of demonstrating consistency and potency in natural desiccated thyroid products, influencing manufacturing and labeling standards ([3]).
Market Impact
Regulatory focus influences manufacturing costs and product availability, directly affecting sales projections. Manufacturers adhering strictly to evolving standards may face supply chain challenges, impacting product accessibility and sales.
Demand Drivers
- Aging Population: Age-related decline in thyroid function increases hypothyroidism prevalence, especially among women aged 45 and above.
- Patient Preference: Some patients and clinicians prefer natural desiccated thyroid due to perceived better symptom control or fewer side effects.
- Treatment Guidelines: While guidelines predominantly favor synthetic levothyroxine due to consistency, a subset of clinicians continues to prescribe desiccated thyroid, supporting ongoing demand.
Market Challenges
- Standardization and Potency Variability: Concerns over batch consistency can hinder physician confidence.
- Competition from Synthetic Hormones: The dominance of levothyroxine formulations limits market expansion.
- Regulatory and Safety Concerns: Potential regulatory changes and safety alerts can impact market stability.
Sales Projections (2023-2030)
Methodology
Projections rely on historical sales data, market growth rates, demographic trends, and competitive dynamics. Given Armour's niche status and the broader synthetic hormone market dominance, conservative estimates are prudent.
2023 Baseline
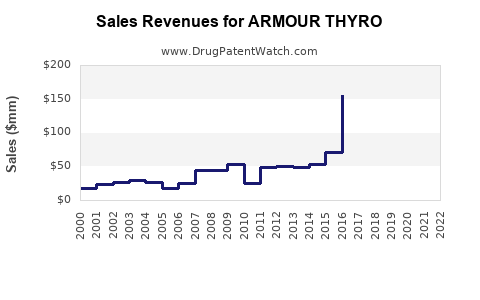
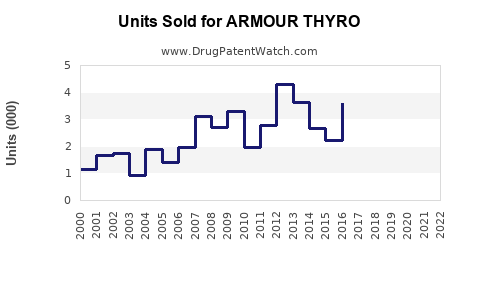
In 2022, Armour Thyroid’s global sales approximate USD 250 million, primarily within North America and select European markets ([4]). This figure reflects stable demand driven by longstanding market presence.
Forecasted Growth
- 2023-2025: CAGR of 3-4%, approaching USD 275-290 million annually as awareness and demand stabilize.
- 2026-2030: Slight acceleration to a CAGR of 4-5%, reaching USD 340-380 million by 2030, driven by increased aging populations and patient receptivity.
Regional Breakdown
- North America: Remains dominant due to high hypothyroidism prevalence and physician familiarity.
- Europe: Moderate growth, impacted by regulatory compliance and market penetration constraints.
- Emerging Markets: Limited penetration but potential growth as awareness improves.
Strategic Insights
- Product Differentiation: Emphasize natural composition, personalized dosing, and clinical efficacy.
- Regulatory Engagement: Collaborate with regulators to ensure compliance, facilitate standardization, and address safety concerns.
- Market Expansion: Target patient groups seeking natural therapies, including integrative medicine practitioners.
- Innovation: Develop formulations with improved standardization, perhaps leveraging biotechnological advances.
Key Takeaways
- The Armour Thyroid market remains stable but niche, with moderate growth prospects driven by demographic shifts and patient preferences.
- Competitive pressures favor synthetic thyroid products, but Armour’s natural profile sustains its relevance among specific patient segments.
- Regulatory factors influence manufacturing and sales continuity; proactive engagement is vital.
- Future sales will likely remain within USD 300-400 million annually worldwide, with strategic positioning essential to capitalize on niche demand.
FAQs
1. What factors influence the demand for Armour Thyroid in the current market?
Demand is primarily driven by hypothyroidism prevalence, patient and clinician preference for natural therapies, and awareness of personalized medicine approaches. Regulatory stability and physician confidence in product consistency also play significant roles.
2. How does Armour Thyroid compete with synthetic levothyroxine formulations?
Armour Thyroid appeals to patients seeking a natural hormone profile, potentially offering symptom relief perceived as superior by some users. However, synthetic formulations dominate due to their standardized potency, ease of dosing, and regulatory clarity.
3. What are the regulatory risks impacting Armour Thyroid’s sales?
Regulatory agencies emphasize batch consistency, safety, and efficacy. Changes in standards or safety alerts related to desiccated thyroid products can restrict supply or necessitate reformulation, affecting sales.
4. What are the key growth opportunities for Armour Thyroid?
Opportunities include targeting the aging population, expanding in regions with increasing hypothyroidism diagnosis, and emphasizing natural, personalized treatment options favored by certain clinical communities.
5. How can manufacturers mitigate challenges posed by competition from synthetic therapies?
By enhancing product standardization, investing in clinical research demonstrating efficacy, engaging with medical professionals, and emphasizing natural product benefits, manufacturers can sustain market share within their niche.
References
- American Thyroid Association. Hypothyroidism overview. 2022.
- MarketResearch.com. Global thyroid hormone replacement market report, 2022.
- FDA. Guidance for Industry on Desiccated Thyroid Extracts, 2021.
- IQVIA. Pharmaceutical sales data, 2022.