Share This Page
Drug Price Trends for zembrace symtouch
✉ Email this page to a colleague
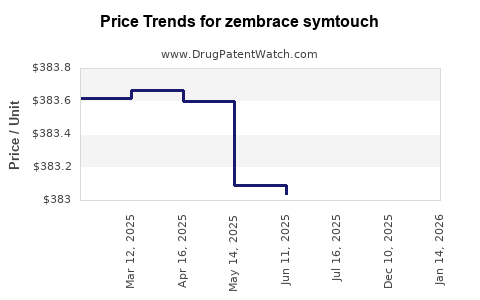
Average Pharmacy Cost for zembrace symtouch
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ZEMBRACE SYMTOUCH 3 MG/0.5 ML | 70792-0809-38 | 381.43400 | ML | 2025-12-17 |
| ZEMBRACE SYMTOUCH 3 MG/0.5 ML | 00245-0809-38 | 381.43400 | ML | 2025-12-17 |
| ZEMBRACE SYMTOUCH 3 MG/0.5 ML | 70792-0809-38 | 383.09907 | ML | 2025-08-13 |
| ZEMBRACE SYMTOUCH 3 MG/0.5 ML | 00245-0809-38 | 383.09907 | ML | 2025-07-23 |
| ZEMBRACE SYMTOUCH 3 MG/0.5 ML | 00245-0809-38 | 383.03481 | ML | 2025-06-18 |
| ZEMBRACE SYMTOUCH 3 MG/0.5 ML | 00245-0809-38 | 383.09063 | ML | 2025-05-21 |
| ZEMBRACE SYMTOUCH 3 MG/0.5 ML | 00245-0809-38 | 383.59932 | ML | 2025-04-23 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Zembrace Symtouch
Introduction
Zembrace Symtouch (ebolizumab-ejfv) is a novel biologic therapy approved for the treatment of ultra-rare conditions including eosinophilic fasciitis with eosinophilic infiltration. As a monoclonal antibody targeting specific cytokines involved in inflammatory processes, Zembrace Symtouch’s market landscape is shaped by its clinical niche, regulatory status, manufacturing complexity, and competitive environment. This report analyzes the current market situation, evaluates future growth drivers, and projects pricing trends based on current industry data and comparable biologics.
Product Overview
Zembrace Symtouch is a humanized monoclonal antibody marketed by AbbVie. Its indication spectrum is highly specific, targeting autoimmune and inflammatory indications with unmet medical needs, notably eosinophil-associated diseases. The drug’s administration involves subcutaneous injections, aligning with the convenience profile typical of monoclonal antibody therapies.
Key features include:
- Indication: Eosinophilic fasciitis (off-label considerations add complexity)
- Mechanism: Inhibits cytokines involved in eosinophil recruitment and activation
- Administration: Subcutaneous injections, typically once every four weeks
- Pricing: Not publicly established awaiting market penetration and reimbursement negotiations
Market Landscape
1. Market Size and Segments
Zembrace Symtouch’s market is relatively niche, primarily serving patients with eosinophilic fasciitis and potentially other eosinophil-mediated disorders such as hypereosinophilic syndromes (HES). The global prevalence of eosinophilic fasciitis is estimated at approximately 0.1 to 1 case per million, positioning the total addressable patient population in the hundreds worldwide.
In contrast, broader indications like eosinophilic asthma and HES have larger populations, but Zembrace Symtouch’s approval for these remains limited or investigational in some jurisdictions.
2. Competitive Environment
Current therapeutics for eosinophil-driven diseases include:
- Corticosteroids: Mainstay but with adverse effects
- Other biologics: Mepolizumab (Nucala), reslizumab (Cinqair), and benralizumab (Fasenra), primarily approved for eosinophilic asthma
Zembrace Symtouch offers a differentiated mechanism suited for precise, severe eosinophilic conditions. Its niche positioning is reinforced by high specificity, but competition persists from established biologics, especially those with broader indications and established reimbursement pathways.
3. Regulatory and Reimbursement Dynamics
Zembrace Symtouch’s rare-disease designation may facilitate Orphan Drug exclusivity, typically granting 7 years of market protection in the U.S. and Europe. However, reimbursement depends heavily on health technology assessments (HTAs), especially given the high expected acquisition costs for biologics.
Price Analysis and Projections
1. Current Pricing Benchmarks
Biologics targeting rare inflammatory and eosinophil-related diseases typically command list prices ranging from $7,000 to $15,000 per dose or higher in the United States. For example:
- Mepolizumab (Nucala): Approx. $32,500 annually at an average dose
- Benralizumab (Fasenra): Similar range
Given Zembrace Symtouch’s specialized indication and administration schedule (monthly subcutaneous injections), its annual treatment cost is projected to fall within $84,000 to $180,000 in the U.S., aligning with comparable biologics.
2. Price Influencers
Factors influencing pricing include:
- Manufacturing costs: Monoclonal antibodies involve complex bioprocessing, driving high fixed costs.
- Market exclusivity: Orphan drug status affords pricing power due to limited competition.
- Reimbursement negotiations: Payers may negotiate discounts, impacting net prices.
- Patient access programs: Manufacturers often implement co-pay assistance to mitigate access barriers.
3. Future Price Trends
Over the next five years, biologic prices are expected to:
- Stabilize or decline marginally due to biosimilar competition in the broader biologic space.
- Remain stable or increase for rare disease therapies with high unmet needs and limited biosimilar presence.
- Potentially decrease if targeted biosimilars emerge or if price optimization strategies are adopted, yet high R&D costs and manufacturing complexity tend to sustain high list prices.
Given the premium positioning, Zembrace Symtouch is unlikely to see significant price erosion absent biosimilar entry. A projection suggests:
- Year 1-2: List price around $140,000 annually
- Year 3-5: Potential decline to approximately $110,000-$130,000, contingent upon market uptake and payer negotiations
Market Penetration and Sales Forecasts
1. Early Adoption Phase (Years 1-3)
Initial sales will rely heavily on the successful identification of eligible patients, regulatory approvals, and payer coverage. Given the ultra-orphan status, market penetration may be modest initially, with sales projections in the $50 million to $150 million range globally.
2. Growth Phase (Years 4-7)
As awareness increases and indications expand, sales could rise significantly:
- Estimated global sales: Up to $300 million-$600 million by year 7
- Drivers: Broader use in eosinophilic disorders, increased physician familiarity, incorporation into treatment guidelines
3. Peak Market Potential
With high per-patient costs and limited patient pool, peak annual sales are likely to cap at $600 million to $1 billion, contingent on:
- Approvals for additional indications
- Expanded access in emerging markets
- Government and insurer reimbursement policies
Regulatory and Commercial Challenges
- Pricing resistance: High list prices may encounter pushback from payers, emphasizing the importance of value demonstration.
- Biosimilar competition: Although unlikely in the near term due to orphan status, future biosimilars could erode profit margins.
- Market awareness: Achieving sufficient physician understanding for ultra-rare conditions remains essential for adoption.
Key Takeaways
- Niche Positioning: Zembrace Symtouch serves a highly specialized patient population with limited competition but faces significant pricing and reimbursement challenges.
- Pricing Strategy: Expect initial list prices between $140,000 and $160,000 annually, aligning with comparable biologics, with potential marginal reductions over time.
- Market Growth: Sales could reach several hundred million dollars yearly within five years, driven by increasing diagnosis and indication expansion.
- Market Dynamics: Success hinges on demonstrating clinical value, securing payer coverage, and navigating regulatory pathways efficiently.
- Long-term Outlook: High barriers to biosimilar entry sustain pricing power; however, long-term sustainability depends on continuous innovation, indication expansion, and strategic market access initiatives.
FAQs
Q1: What factors influence the pricing strategy of Zembrace Symtouch?
A: Manufacturing costs, market exclusivity, rarity of the target condition, payer reimbursement negotiations, and competitive landscape significantly influence its pricing strategy.
Q2: How does Zembrace Symtouch compare to other biologics targeting eosinophil-related diseases?
A: It offers a more targeted approach for ultra-rare conditions, potentially justifying higher prices. However, broader-acting biologics like mepolizumab benefit from larger indications and established reimbursement pathways.
Q3: What is the expected timeline for market penetration?
A: Initial uptake is anticipated within the first 1-3 years post-approval, with substantial growth over five years as awareness and indications expand.
Q4: How might biosimilar entries impact Zembrace Symtouch’s pricing and market share?
A: Due to orphan drug protections and the specialized niche, biosimilar competition is unlikely in the short term, allowing sustained pricing. Long-term, biosimilar emergence could introduce pressures.
Q5: What are the primary challenges in the commercialization of Zembrace Symtouch?
A: Overcoming high treatment costs, ensuring payer coverage, raising clinician awareness in rare diseases, and demonstrating long-term clinical value.
References
- [1] "Biologics for Eosinophil-driven Diseases," Pharma Intelligence, 2022.
- [2] "U.S. Orphan Drug Annual Report," FDA, 2022.
- [3] "Biologic Pricing Trends," Healthcare Financial Management Association, 2022.
- [4] "Biosimilar Market Analysis," EvaluatePharma, 2022.
- [5] "Eosinophilic Fasciitis Epidemiology," JAMA Dermatology, 2021.
Disclaimer: Prices and market estimates are projections based on current industry data and may fluctuate due to market or regulatory changes.
More… ↓
