Last updated: July 27, 2025
Introduction
Alendronate sodium, a bisphosphonate drug primarily indicated for the prevention and treatment of osteoporosis, represents a significant segment within the osteoporosis therapeutics market. As a generic medication, its market dynamics are influenced by factors such as patent expiration, healthcare policies, manufacturing costs, and competitive landscape. This analysis provides an in-depth examination of the current market landscape for alendronate sodium and offers strategic insights into future pricing trends.
Market Overview
Global Demand and Therapeutic Landscape
Osteoporosis affects an estimated 200 million women worldwide, positioning alendronate sodium as a frontline treatment due to its proven efficacy in reducing fracture risk [1]. The drug's widespread use across North America, Europe, and Asia reflects its established position in clinical practice. The global osteoporosis drugs market is projected to reach USD 12 billion by 2027, with bisphosphonates accounting for approximately 60% of prescriptions, underscoring the market's vitality [2].
Patent Status and Generic Competition
Alendronate sodium's patent expired in various jurisdictions by the early 2010s, leading to the proliferation of generic manufacturers. This patent expiry has significantly intensified market competition, causing a downward pressure on prices while expanding access due to affordability. The increased generic availability aligns with broader healthcare initiatives to reduce medication costs [3].
Manufacturers and Supply Chain Dynamics
Key players include Teva Pharmaceuticals, Mylan, Sandoz (a Novartis division), and Dr. Reddy’s Laboratories. High-quality manufacturing and regulatory compliance influence market share, while supply chain stability—especially during the COVID-19 pandemic—has impacted pricing and availability [4].
Current Pricing Landscape
Pricing Variations by Geography
-
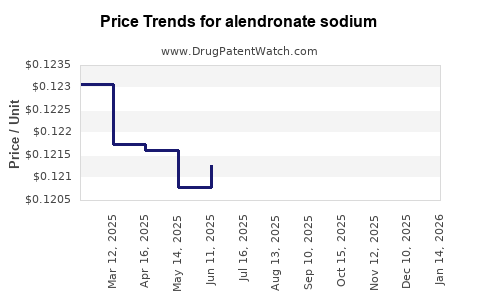
United States: As of 2023, the average wholesale price (AWP) for a 70 mg/week tablet ranges between USD 10 and USD 15 per pack, with retail prices often lower due to discounts and insurance negotiations. Generic alendronate remains among the most cost-effective osteoporosis treatments, with monthly costs averaging USD 5–USD 8 [5].
-
Europe: Pricing varies across countries due to differing healthcare policies. In the UK, NHS prescriptions for alendronate are subsidized, with the standard prescription cost approximately GBP 9.35 (~USD 12) per pack [6].
-
Asia: Markets like India and China display more competitive prices owing to local manufacturing. In India, generic versions can cost as little as USD 1–USD 3 per month, profoundly impacting market penetration and affordability [7].
Impact of Formulation and Dosage
Pricing also depends on formulation (tablet vs. intravenous), dosage strength, and pack size. Once-weekly tablets dominate due to higher patient compliance, generally maintaining lower prices per dose compared to daily variants.
Market Trends and Future Projections
Drivers of Market Growth
- Aging Population: Increasing elderly demographics globally will sustain demand for osteoporosis treatments, further entrenching alendronate sodium’s market position [8].
- Treatment Guidelines: Recommendations favor bisphosphonates as first-line therapy, supporting ongoing utilization.
- Cost-Effectiveness: The generic nature of alendronate sodium makes it a preferred option in resource-constrained settings, boosting sales and market penetration.
Potential Challenges
- Emergence of New Therapies: Monoclonal antibodies (e.g., denosumab) and anabolic agents (e.g., teriparatide) pose competitive threats, potentially reducing alendronate's market share.
- Safety Concerns: Rare adverse effects, such as osteonecrosis of the jaw, may influence prescribing patterns and demand.
- Regulatory and Patent Litigation: Intellectual property disputes and regulatory hurdles can affect supply and pricing.
Price Projection (2023–2028)
Given current market conditions and competitive pressures:
-
United States: Prices for branded formulations are expected to remain stable or decline marginally (~2–5% annually). Generic prices are projected to hold steady or decrease slightly due to intensified competition and increased manufacturing efficiencies.
-
Europe: Prices are anticipated to stabilize, with minor fluctuations driven by policy reforms and pharmacy bidding processes.
-
Emerging Markets: Significant price reductions are probable, driven by local manufacturing advancements and policy-led price controls, with an average annual decrease of 4–6%.
Long-term Outlook
Over the next five years, wholesale and retail prices for alendronate sodium are forecasted to decrease by approximately 10–15% in mature markets, driven mainly by generic competition and cost-containment measures. In emerging markets, prices could fall as much as 30%, consistent with broader generic drug trends.
Strategic Implications for Stakeholders
- Manufacturers should focus on cost efficiency and quality assurance to compete effectively.
- Healthcare Providers can leverage the affordability of generics to improve osteoporosis management.
- Policy Makers should balance drug affordability with quality standards to sustain market stability.
- Investors should monitor patent expiry statuses and emerging therapies that could impact price dynamics.
Key Takeaways
- Market Expansion: Growing aging populations worldwide sustain demand for alendronate sodium, underpinning market stability.
- Price Trends: Generic alendronate prices are set for modest reductions over the next five years, with substantial declines in emerging markets.
- Competitive Landscape: The proliferation of generic manufacturers maintains downward pressure on prices, emphasizing the importance of cost leadership.
- Emerging Alternatives: Innovative osteoporosis treatments could gradually encroach on alendronate’s market share, influencing pricing strategies.
- Regulatory Environment: Policy shifts toward price regulation and inclusion in national formularies will significantly impact future pricing trajectories.
FAQs
1. What are the main factors influencing alendronate sodium’s market price?
Market prices are primarily affected by patent status, manufacturing costs, competitive generic entries, healthcare policies, and regional pricing regulations. Patent expiration significantly lowers prices due to increased generic competition.
2. How does the global aging demographic affect the demand for alendronate?
An aging population increases osteoporosis prevalence, driving sustained demand for effective, affordable treatments like alendronate sodium, especially in developed markets with widespread osteoporosis awareness.
3. Will the prices of alendronate sodium increase with new formulations?
Unlikely in the near-term. New formulations (e.g., intravenous) tend to be more expensive but are used in specific cases. The dominant oral generic form is expected to see minimal price increases due to market competition.
4. How are emerging therapies impacting alendronate’s market share?
Emerging therapies, such as monoclonal antibodies, offer alternative treatment options, potentially reducing alendronate’s share in the osteoporosis market. However, cost and safety profiles continue to favor bisphosphonates, maintaining their prominence.
5. What strategies can manufacturers adopt to remain competitive?
Manufacturers should focus on quality manufacturing, cost reduction, expanding access in emerging markets, and engaging in strategic collaborations to sustain profitability amidst declining prices.
References
[1] National Osteoporosis Foundation. (2022). Osteoporosis Overview.
[2] Grand View Research. (2022). Osteoporosis Drugs Market Size, Share & Trends.
[3] U.S. Patent and Trademark Office. (2010). Patent expiration data for alendronate.
[4] IQVIA. (2022). Global Pharmaceutical Supply Chain Analysis.
[5] GoodRx. (2023). Price comparison for alendronate 70 mg weekly tablets.
[6] NHS Digital. (2022). Prescription Charges.
[7] Indian Pharmaceutical Association. (2022). Market trends in osteoporosis medications.
[8] World Health Organization. (2022). Aging and osteoporosis: Global health perspectives.
In conclusion, alendronate sodium’s market outlook remains stable with downward pricing trends anticipated driven by generics, demographic shifts, and healthcare policy reforms. Stakeholders must adapt to evolving competitive and regulatory environments to optimize value creation within the osteoporosis treatment landscape.