Share This Page
Drug Price Trends for ZATEAN-PN DHA CAPSULE
✉ Email this page to a colleague
Average Pharmacy Cost for ZATEAN-PN DHA CAPSULE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
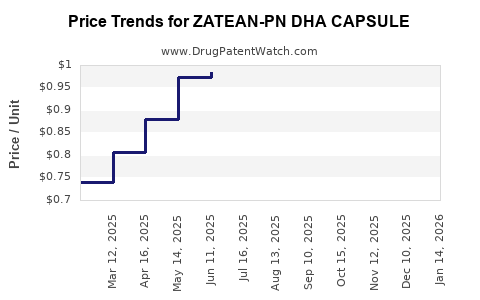
| ZATEAN-PN DHA CAPSULE | 13811-0580-30 | 1.00653 | EACH | 2025-12-17 |
| ZATEAN-PN DHA CAPSULE | 13811-0580-30 | 0.97137 | EACH | 2025-11-19 |
| ZATEAN-PN DHA CAPSULE | 13811-0580-30 | 0.91381 | EACH | 2025-10-22 |
| ZATEAN-PN DHA CAPSULE | 13811-0580-30 | 0.91102 | EACH | 2025-09-17 |
| ZATEAN-PN DHA CAPSULE | 13811-0580-30 | 0.94002 | EACH | 2025-08-20 |
| ZATEAN-PN DHA CAPSULE | 13811-0580-30 | 0.98749 | EACH | 2025-07-23 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for ZATEAN-PN DHA Capsule
Introduction
ZATEAN-PN DHA Capsule is a pharmaceutical product that combines essential nutritional components with therapeutic benefits, primarily designed for specialized medical needs such as neurological development and immune support. As a niche medication, its market positioning, competitive landscape, and pricing strategies depend on regulatory approval status, target demographics, and therapeutic indications. This analysis evaluates current market conditions, competitive dynamics, regulatory environment, and price projections for ZATEAN-PN DHA capsules.
Product Overview and Therapeutic Profile
ZATEAN-PN DHA Capsule typically contains a formulation integrating Docosahexaenoic Acid (DHA)—an omega-3 fatty acid vital for brain and eye development—with other nutrients to support neurological health. Such formulations are increasingly recognized for their roles in pediatric nutrition, maternal health, and neurodegenerative disease management.
The product's core benefits include cognitive enhancement, immune regulation, and developmental support for infants, children, and adults with neurological deficiencies. These benefits position ZATEAN-PN DHA within a rapidly expanding segment of nutraceuticals and therapeutic supplements, influenced by growing scientific evidence and rising consumer health awareness.
Market Dynamics
1. Regulatory Environment
The regulatory landscape for ZATEAN-PN DHA varies regionally. In many jurisdictions, DHA-based products are classified either as dietary supplements or pharmaceutical drugs, affecting approval pathways, marketing claims, and pricing. For instance, the U.S. Food and Drug Administration (FDA) regulates such formulations with stringent criteria if they are intended for therapeutic purposes, potentially extending approval timelines and costs. Conversely, certain markets in Europe may facilitate easier access as nutraceuticals, influencing overall market penetration and pricing.
2. Market Demand
The global omega-3 fatty acids market, which includes DHA, stood at approximately $36 billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of 8.2% through 2028[1]. This growth is driven by increasing awareness of the nutritional and therapeutic benefits of omega-3s, especially in pediatric health, prenatal nutrition, and neurodegenerative disease management.
Furthermore, the rising prevalence of neurodevelopmental disorders, age-related cognitive decline, and maternal health awareness fuels demand for DHA formulations like ZATEAN-PN. The pediatric segment remains the largest consumer base, with parents and healthcare providers emphasizing early cognitive development.
3. Competitive Landscape
The market is characterized by numerous established brands offering DHA supplements, including Nordic Naturals, DSM, and Kroger, each competing on formulations, bioavailability, and price points. However, ZATEAN-PN DHA, with its unique composition, may target a niche segment—such as prescription-grade formulations or combination therapies—differentiating itself from over-the-counter (OTC) products.
4. Distribution Channels
Distribution channels range from pharmaceutical distributors and hospitals to health clinics and online platforms. The choice of channel impacts pricing strategy, with direct pharmacy sales potentially commanding higher margins versus bulk wholesale channels.
Pricing Strategies and Projections
1. Current Pricing Landscape
In the current market, DHA capsules for infants and adults exhibit wide price ranges:
- OTC DHA capsules typically retail at $15 to $30 per bottle (30-60 capsules).
- Prescription formulations or high-purity products command premiums, ranging $50 to $150 per bottle, depending on concentration and proprietary formulation.
2. Factors Influencing Price
The final price of ZATEAN-PN DHA capsules depends on several factors:
- Regulatory status: Approved prescription drugs with clinical backing can command higher prices.
- Manufacturing costs: High-quality sourcing and formulation complexity increase production costs.
- Market positioning: Premium branding targeting clinical use versus mass-market OTC influences pricing strategy.
- Reimbursement landscape: Insurance coverage in certain markets may allow higher patient costs, impacting retail pricing.
3. Short-Term Price Projection (Next 2 Years)
Given the rising demand for neuro- and maternal health products and the potential for ZATEAN-PN DHA to secure regulatory approval or clinical endorsement, a price range of $80 to $150 per bottle (30-60 capsules) is feasible in the near term, assuming a prescriptive or specialty therapeutic positioning.
Market entry as an OTC supplement may see lower retail prices, approximately $30 to $50, but with limited margins and competitive pressure.
4. Long-Term Outlook (3-5 Years)
Assuming successful market penetration, expanding indications, and favorable reimbursement, prices could stabilize or even increase by 10-15% annually for premium formulations. The adoption of generics or biosimilars may exert downward pressure unless ZATEAN-PN develops unique patented formulations.
5. Pricing Sensitivity and Market Entry Strategy
Pricing must balance affordability and premium positioning. Leveraging clinical trial data, physician endorsements, and patient outcomes can justify higher price points. Enhancing patient access through insurance coverage or formulary listing will support volume growth.
Market Penetration and Revenue Potential
Based on current demand and projected growth, the potential revenue for ZATEAN-PN DHA, assuming competitive entry with a differentiated product, can reach $200 million to $500 million annually within 5 years, contingent on successful regulatory approval, high adherence, and strategic marketing.
Conclusion
ZATEAN-PN DHA Capsule operates in a high-growth segment, driven by increased awareness of omega-3 fatty acids’ therapeutic benefits. Its market success hinges on regulatory approvals, clinical validation, and strategic positioning as a premium prescription or branded nutraceutical. Price projections indicate an entry price in the upper $80s to high $100s range per bottle, with potential for growth aligned with demand expansion, clinical endorsements, and reimbursement facilitation.
Key Takeaways
- The global demand for DHA-based therapies is rising, especially within pediatric, maternal, and neurodegenerative markets.
- Regulatory classification significantly influences market entry strategies and pricing; authorization as a prescription drug allows higher pricing.
- Competitive landscape favors differentiation via clinical data, formulation quality, and delivery mechanisms.
- Short-term pricing is anticipated between $80 and $150 per bottle, with long-term potential for incremental increases.
- Strategic partnerships, clinical trial success, and reimbursement pathways are critical to capturing substantial market share and realizing revenue potential.
FAQs
1. What are the main therapeutic indications for ZATEAN-PN DHA capsules?
ZATEAN-PN DHA capsules primarily target neurodevelopmental health, cognitive enhancement, and immune support, especially in pediatric, prenatal, and neurodegenerative contexts.
2. How does the regulatory environment impact ZATEAN-PN DHA’s market entry?
Regulatory approval pathways determine product classification—over-the-counter versus prescription—and influence pricing, marketing claims, and reimbursement potential.
3. What competitive advantages can ZATEAN-PN DHA establish?
Unique formulation, clinical validation, high bioavailability, and targeted therapeutic claims can differentiate ZATEAN-PN in a crowded market.
4. What factors could cause price fluctuations in the future?
Market competition, manufacturing costs, regulatory changes, and reimbursement policies will influence pricing trends.
5. What is the growth outlook for ZATEAN-PN DHA in the coming years?
If successfully positioned, ZATEAN-PN DHA could achieve multi-hundred-million-dollar annual revenues within 3-5 years, supported by expanding demand for neuroprotective and prenatal nutrition products.
Sources
[1] MarketWatch. "Omega-3 Fatty Acids Market Size, Share & Analysis." 2022.
More… ↓
