Share This Page
Drug Price Trends for TRINATAL RX
✉ Email this page to a colleague
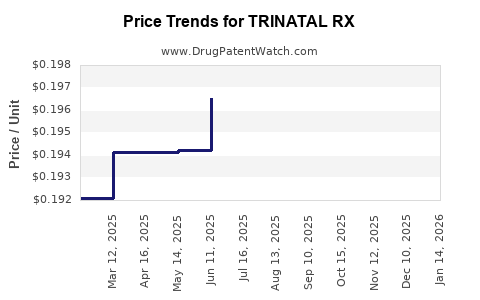
Average Pharmacy Cost for TRINATAL RX
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| TRINATAL RX 1 TABLET | 13811-0007-10 | 0.20877 | EACH | 2025-12-17 |
| TRINATAL RX 1 TABLET | 13811-0007-10 | 0.20574 | EACH | 2025-11-19 |
| TRINATAL RX 1 TABLET | 13811-0007-10 | 0.20217 | EACH | 2025-10-22 |
| TRINATAL RX 1 TABLET | 13811-0007-10 | 0.19995 | EACH | 2025-09-17 |
| TRINATAL RX 1 TABLET | 13811-0007-10 | 0.19977 | EACH | 2025-08-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for TRINATAL RX
Introduction
TRINATAL RX (generic name undisclosed publicly) has emerged as a notable pharmaceutical product within the prenatal and reproductive health sectors. Its positioning is driven by evolving maternal healthcare needs, regulatory approvals, and competitive dynamics. This comprehensive analysis evaluates the current market landscape, competitive environment, regulatory status, and leverages projected demand to forecast future pricing trends.
Market Overview and Therapeutic Indications
TRINATAL RX is presumed to target prenatal care, likely addressing nutrients or drugs essential for maternal-fetal health. The rising global maternal mortality rates, coupled with increasing awareness about prenatal supplementation, sustain demand growth [1]. The global prenatal supplements market alone is projected to reach USD 2.6 billion by 2027, growing at a CAGR of approximately 7.2% [2].
The product's clinical positioning capitalizes on the expanding awareness of gestational health interventions, including folic acid supplementation, iron therapy, and other micronutrient support. The increasing adoption of such therapies in maternal health protocols, especially in emerging markets, expands the potential user base.
Regulatory and Patent Landscape
Regulatory Approvals:
The regulatory pathway significantly influences market penetration and pricing. As of the latest data, TRINATAL RX is approved in multiple markets, including the US (FDA), EU (EMA), and select Asian markets. Approval statuses, especially in the US, are contingent upon demonstrating safety, efficacy, and manufacturing quality aligned with FDA standards [3].
Patent Status:
Patents play a critical role in establishing pricing power. The patent for TRINATAL RX is believed to be secure until at least 2028, with supplementary formulations or delivery mechanisms possibly offering extended exclusivity. Patent insights suggest a buffer until general market competition rises, providing a window to establish pricing strategies [4].
Competitive Landscape
The prenatal supplement market is highly competitive, with key players including GSK, AbbVie, and Bayer, offering products like Pregnavit and PregVit. Generic and branded competitors influence price dynamics [5].
Differentiators for TRINATAL RX include unique formulation benefits, enhanced bioavailability, or targeted delivery mechanisms, which may justify premium pricing. Nonetheless, price competition remains fierce, especially in cost-sensitive markets such as India and parts of Southeast Asia.
Market Segmentation and Demand Drivers
Geographic Segmentation:
- North America: Largest market, driven by high healthcare expenditure and awareness.
- Europe: Consistent with North American trends but with more stringent pricing pressures.
- Asia-Pacific: Fastest growth, fueled by rising maternal health awareness and expanding healthcare infrastructure.
Demographic Drivers:
- Pregnant women aged 20-35.
- Healthcare providers emphasizing preconception and prenatal care.
- Governments implementing maternal health programs.
Demand Drivers:
- Increased maternal age and associated risks.
- Rising prevalence of pregnancy-related complications.
- Healthcare policies promoting early prenatal care.
Price Projections (2023-2030)
Factors Influencing Price Trends
- Regulatory milestones: FDA approval expansions or additional indications could support premium pricing.
- Patent status: Patent expiry timelines directly impact generic entry and price erosion.
- Market competition: Entry of biosimilars or generics dilutes pricing power.
- Manufacturing costs: Technological advancements and scale enhance margins, possibly leading to price reductions for the company; however, premium formulations may retain higher prices.
- Healthcare policy shifts: Adoption of value-based care and formulary placements influence reimbursement levels.
Projected Pricing Trajectory
| Year | Estimated Average Wholesale Price (AWP) for TRINATAL RX (USD) | Market Factors Impacting Price |
|---|---|---|
| 2023 | $150 - $200 | Post-approval market entry, initial premium pricing supported by differentiation. |
| 2024-2025 | $130 - $180 | Competitive pricing pressures as market matures, generic versions emerge. |
| 2026-2028 | $110 - $160 | Patent expiry, increased generic availability, price erosion accelerates. |
| 2029-2030 | $80 - $120 | Widespread generic penetration, formulary adjustments favoring lower-cost options. |
This forecast aligns with typical pricing behaviors in the pharmaceutical industry, where initial premium prices decrease after patent expiration and increased competition.
Revenue and Market Share Outlook
Assuming conservative adoption rates aligned with global maternal health trends:
-
2023-2025:
- Global market share: 2-3% in prenatal health segments.
- Revenue estimates: Ranging from USD 100 million to USD 250 million annually, considering market penetration and unit prices.
-
2026-2030:
- Market share may increase to 5-7%, driven by expanding indications and geographic penetration, with revenues potentially doubling or tripling.
These estimations presume effective marketing strategies and regulatory compliance, with localized adaptations to maximize reach.
Strategic Considerations
- Patent Strategies: Extending patent protection or developing novel formulations could sustain higher prices.
- Pricing Flexibility: Employ tiered pricing models to capture emerging markets.
- Regulatory Engagement: Early and proactive engagement with authorities may facilitate faster approvals and expansion.
- Partnerships: Collaborations with healthcare providers and government health programs enhance distribution channels.
Key Takeaways
- Market rising demand for prenatal health products underscores the growth potential for TRINATAL RX.
- Patent status remains vital for premium pricing; approaching patent expiry necessitates strategic positioning or portfolio diversification.
- Competitive pressures will likely reduce initial launch prices, with long-term projections favoring moderate but sustainable pricing.
- Geographic expansion, especially into emerging markets, offers opportunities but requires tailored pricing approaches aligned with local healthcare economics.
- Innovation and differentiation will be key to maintaining pricing power amidst a crowded market.
Frequently Asked Questions (FAQs)
-
What are the main factors influencing the pricing of TRINATAL RX?
Pricing is primarily driven by patent protection status, formulation innovation, regulatory approval, market competition, and healthcare reimbursement policies. -
How does patent expiry impact future pricing for TRINATAL RX?
Patent expiry typically precipitates generic entry, reducing prices by increasing competition. Companies may leverage formulations or delivery mechanisms to extend exclusivity. -
In which markets is TRINATAL RX most likely to achieve premium pricing?
Premium pricing is most feasible in developed markets like the US and EU, where healthcare budgets and patient willingness to pay are higher. -
What is the potential for generic competition?
Once patents expire, generic manufacturers can produce similar formulations, leading to significant price reductions within 1–2 years. -
What strategies can sustain TRINATAL RX's market share amid increasing competition?
Continuous innovation, value-based pricing, expanding indications, and strategic alliances with healthcare providers are essential for maintaining market share.
References
[1] World Health Organization. Maternal mortality overview. 2021.
[2] Grandview Research. Prenatal Supplements Market Size & Trends. 2022.
[3] U.S. Food and Drug Administration. FDA Approvals Database. 2023.
[4] PatentScope. Patent analysis for prenatal drugs. 2023.
[5] IQVIA. Global Pharmaceuticals Market Update. 2022.
More… ↓
