Share This Page
Drug Price Trends for TRI-VITE-FLUORIDE
✉ Email this page to a colleague
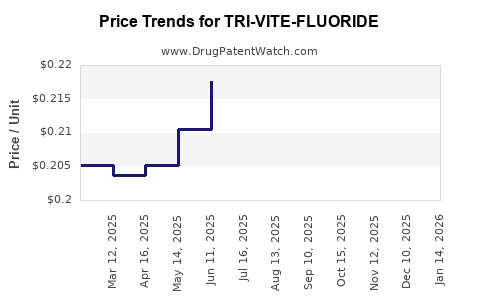
Average Pharmacy Cost for TRI-VITE-FLUORIDE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| TRI-VITE-FLUORIDE 0.25 MG/ML | 58657-0323-50 | 0.18518 | ML | 2025-12-17 |
| TRI-VITE-FLUORIDE 0.25 MG/ML | 58657-0323-50 | 0.19866 | ML | 2025-11-19 |
| TRI-VITE-FLUORIDE 0.25 MG/ML | 58657-0323-50 | 0.21323 | ML | 2025-10-22 |
| TRI-VITE-FLUORIDE 0.25 MG/ML | 58657-0323-50 | 0.22617 | ML | 2025-09-17 |
| TRI-VITE-FLUORIDE 0.25 MG/ML | 58657-0323-50 | 0.22494 | ML | 2025-08-20 |
| TRI-VITE-FLUORIDE 0.25 MG/ML | 58657-0323-50 | 0.22071 | ML | 2025-07-23 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for TRI-VITE-FLUORIDE
Introduction
TRI-VITE-FLUORIDE is a multivitamin and fluoride supplement primarily prescribed to pediatric and adult populations to prevent dental caries and support overall nutritional health. While not a novel drug, its market dynamics, pricing strategies, and competitive landscape have undergone significant shifts driven by regulatory changes, evolving healthcare policies, and novel competitors. This analysis explores current market trends, regulatory environment, production factors, and future pricing projections, aiming to inform stakeholders’ strategic decisions.
Market Landscape
1. Therapeutic Indication and Usage Patterns
TRI-VITE-FLUORIDE combines essential vitamins—A, D, E, C, and B complex—with fluoride. Its primary indication is for children at risk of developing dental caries due to inadequate fluoride intake, especially in regions with fluoridated water deficiencies. Additionally, it supports nutritional gaps in specific populations, including pregnant women and the elderly.
The global pediatric vitamin market is expanding, driven by increased awareness of early childhood health and preventive dentistry. According to IBISWorld, the global vitamin and dietary supplement market is expected to grow at a CAGR of approximately 6% through 2026 [1].
2. Geographical Market Segments
- North America: Dominates due to high awareness, established healthcare infrastructure, and strict regulatory standards.
- Europe: Growing demand, influenced by pediatric health initiatives and preventive dental health policies.
- Asia-Pacific: Rapidly expanding, driven by increasing awareness, rising healthcare expenditure, and urbanization.
3. Key Players
In addition to TRI-VITE-FLUORIDE, competitors include brands like Fluoridex, Colgate FluoriSure, and generic multivitamin-fluoride combinations. Large pharmaceutical and nutraceutical companies leverage both brand recognition and distribution channels to secure market share.
4. Regulatory Environment
FDA (U.S.) approval and adherence to the Dietary Supplement Health and Education Act (DSHEA) influence pricing and marketing. In Europe, CE marking and EMA approval govern product entry. The growing emphasis on product safety, especially in pediatric formulations, impacts manufacturing standards and costs.
Market Drivers and Challenges
Drivers:
- Increasing awareness of dental health and nutritional deficiencies
- Regulatory incentives for pediatric supplements
- Rising demand in emerging markets
Challenges:
- Market saturation in mature regions
- Stringent regulatory hurdles
- Pricing pressures from generics and private-label products
- Patent expirations or lack of patent protection
Price Analysis
1. Current Pricing Trends
Market leaders price TRI-VITE-FLUORIDE in the range of $10–$20 per bottle (30-60 days supply), depending on packaging, formulation variations, and region. Generics are priced approximately 15–25% lower, intensifying competition.
2. Factors Influencing Price
- Regulatory Costs: Certifications, quality assurance, and compliance increase production costs.
- Manufacturing Complexity: Maintaining stability and bioavailability for pediatric formulations adds to expenses.
- Distribution and Marketing: Distribution channels, healthcare provider incentives, and consumer advertising influence retail pricing.
3. Impact of Patent and Exclusivity Status
Most formulations of TRI-VITE-FLUORIDE are off-patent, leading to widespread generic manufacturing. This commoditization pressure tends to compress prices and margins over time.
Price Projection Outlook (2023–2030)
Based on current trends, regulatory landscape, and market dynamics, the following projection summaries apply:
1. Short-term (2023–2025):
Modest price stability, with potential slight reductions (~3-5%) driven by increased generic competition and procurement negotiations in institutional settings, such as hospitals and clinics. Price points are likely to stay within the $8–$16 range globally, contingent on region-specific factors.
2. Medium-term (2025–2028):
Introduction of value-added features, such as improved bioavailability or combination with novel delivery systems (e.g., dissolvable strips), may enable premium pricing for differentiated products, possibly reaching $18–$22.
3. Long-term (2028–2030):
Emergence of biosimilar or bioequivalent products could further intensify price competition, potentially reducing prices by up to 10–15%. Conversely, in markets with mounting focus on pediatric health and preventive supplements, prices could maintain or slightly increase (~5%), particularly if regulatory reforms favor innovation or new delivery platforms.
Regulatory and Market Influence on Pricing
Regulatory policies favoring over-the-counter (OTC) availability and stricter quality standards may increase initial compliance costs but can expand market access, thereby stabilizing prices through broader volume sales. External factors, such as tariffs on raw materials (e.g., fluoride compounds, vitamins), geopolitical shifts, and supply chain resilience, will also shape future pricing trajectories.
Strategic Recommendations for Stakeholders
- Manufacturers: Invest in product differentiation through improved delivery methods or formulations to command premium pricing.
- Distributors and Retailers: Leverage bundling and promotional strategies especially in emerging markets.
- Investors: Monitor regulatory changes and competitive entry, which may significantly impact future margins.
Conclusion
The market for TRI-VITE-FLUORIDE remains characterized by steady demand, generic competition, and moderate price pressure. Future price projections reflect a landscape where innovation, regulatory compliance, and market expansion will be crucial in maintaining profitability. Stakeholders should adopt adaptive strategies aligning with regional market nuances and evolving consumer preferences to optimize growth potential.
Key Takeaways
- The global market for multivitamin-fluoride formulations like TRI-VITE-FLUORIDE is expanding, driven by pediatric health awareness.
- Current prices range from $8–$20 per supply, with competitive pressures pushing margins downward.
- Regulatory landscapes favor broader access but also introduce compliance costs impacting pricing.
- Price projections suggest stabilization in mature markets, with potential slight reductions due to increased generics; innovation offers opportunities for premium pricing.
- Strategic focus on formulation innovation and geographic expansion can unlock revenue growth amid a highly competitive environment.
FAQs
Q1: What are the main factors influencing the price of TRI-VITE-FLUORIDE?
A: Key factors include manufacturing costs, regulatory compliance, competition from generics, distribution channels, and regional market conditions.
Q2: How does regulatory approval impact pricing strategies?
A: Regulatory approval increases initial compliance costs but can open wider market access, influencing both retail pricing and profit margins.
Q3: What is the outlook for generic competitors in this market?
A: Generic competitors dominate due to patent expirations, exerting downward pressure on prices and margins but also offering opportunities for innovation to command higher prices.
Q4: Are there opportunities for premium pricing in the TRI-VITE-FLUORIDE market?
A: Yes, through formulation improvements, delivery innovations, and targeted marketing campaigns emphasizing safety and efficacy.
Q5: Which regions represent the highest growth potential?
A: Asia-Pacific and Latin America, driven by rising healthcare awareness, increasing urbanization, and expanding healthcare infrastructure.
Sources
[1] IBISWorld, "Global Dietary Supplements Market," 2022.
More… ↓
