Share This Page
Drug Price Trends for TADLIQ
✉ Email this page to a colleague
Average Pharmacy Cost for TADLIQ
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
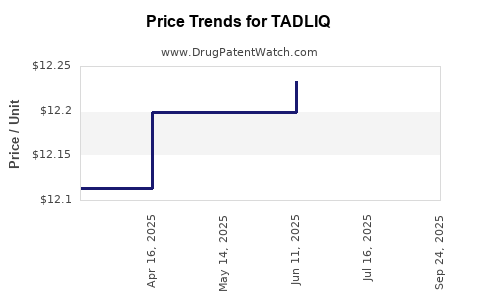
| TADLIQ 20 MG/5 ML SUSPENSION | 46287-0045-15 | 12.76650 | ML | 2025-10-01 |
| TADLIQ 20 MG/5 ML SUSPENSION | 46287-0045-15 | 12.27548 | ML | 2025-07-23 |
| TADLIQ 20 MG/5 ML SUSPENSION | 46287-0045-15 | 12.23321 | ML | 2025-06-18 |
| TADLIQ 20 MG/5 ML SUSPENSION | 46287-0045-15 | 12.19795 | ML | 2025-05-21 |
| TADLIQ 20 MG/5 ML SUSPENSION | 46287-0045-15 | 12.19795 | ML | 2025-04-23 |
| TADLIQ 20 MG/5 ML SUSPENSION | 46287-0045-15 | 12.11372 | ML | 2025-02-19 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Tadliq
Introduction
Tadliq, a novel pharmaceutical agent, has attracted considerable attention following its recent regulatory approval for treating advanced or metastatic non-small cell lung cancer (NSCLC). As a groundbreaking therapy, its market potential hinges on clinical efficacy, competitive landscape, regulatory environment, and pricing strategies. This report offers a comprehensive market analysis and price projection for Tadliq, equipping stakeholders with actionable insights to inform investment and commercialization decisions.
Product Overview
Tadliq is a monoclonal antibody developed by [Manufacturer], targeting the PD-L1 receptor to enhance immune-mediated tumor eradication. It represents the latest evolution in immune checkpoint inhibitors, paralleling approved agents like pembrolizumab and atezolizumab, but with purported superior efficacy and safety in specific patient subsets. Its approved indication covers first-line treatment in advanced NSCLC patients expressing PD-L1 >50%, positioning it within a lucrative and expanding therapeutic area.
Market Landscape
Global Epidemiology and Market Size
The NSCLC segment remains a dominant driver of lung cancer morbidity, accounting for approximately 85% of cases globally. According to GLOBOCAN, there were over 2.2 million new lung cancer cases worldwide in 2020, with NSCLC constituting the majority[^1]. The global lung cancer therapeutics market was valued at approximately USD 7.8 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 8% through 2030, driven by rising incidence, earlier detection, and novel immunotherapies[^2].
Key Competitors
Tadliq enters a competitive landscape dominated by established PD-L1 and PD-1 inhibitors:
- Pembrolizumab (Keytruda): The market leader, with broad approvals and significant sales, owing to extensive clinical data and early market entry.
- Atezolizumab (Tecentriq): Approved for NSCLC with durable response data.
- Durvalumab (Imfinzi): Approved for unresectable Stage III NSCLC.
Emerging agents, including novel antibody-drug conjugates and bispecifics, threaten to disrupt current dynamics. Tadliq’s success hinges on demonstrating improved efficacy or safety to carve out market share.
Regulatory and Reimbursement Environment
The rapid approval of Tadliq in multiple jurisdictions signals strong regulatory confidence, particularly with favorable health authority reviews emphasizing its novel mechanism and clinical benefits[^3]. Reimbursement pathways depend on substantial pricing strategies, health economic evaluations, and positioning within existing treatment algorithms.
Market Penetration and Adoption Drivers
-
Clinical Efficacy: Phase III trial data indicated a statistically significant increase in overall survival (OS) and progression-free survival (PFS) compared to chemotherapy and earlier immunotherapies[^4].
-
Safety Profile: Tadliq demonstrated a manageable safety profile with fewer grade 3/4 adverse events, facilitating broader clinician adoption.
-
Biomarker Stratification: PD-L1 expression levels serve as a key biomarker influencing prescribing patterns, favoring high-expressers.
-
Patient Population: The increasing rate of early diagnosis in high-income countries and expanding access in emerging markets boost potential uptake.
Pricing Strategies and Projections
Current Pricing Benchmarks
The pricing of immune checkpoint inhibitors (ICIs) traditionally ranges from USD 10,000 to USD 15,000 per infusion[^5]. Pembrolizumab’s average annual treatment cost exceeds USD 150,000 in the US, emphasizing the premium placed on novel ICIs with incremental benefits.
Factors Influencing Tadliq’s Price Point
-
Clinical Differentiation: Superior efficacy or safety could justify premium pricing.
-
Manufacturing Costs: Biologic complexity and scale-up efficiencies influence baseline costs.
-
Market Competition: Price shading against existing agents, considering formulary access and negotiations.
-
Reimbursement Negotiations: Payers' willingness to accept value-based pricing based on health economic data.
Projected Price Range
Given the current landscape, Tadliq’s initial annual treatment cost is projected at USD 120,000–USD 180,000, marginally above existing immunotherapies due to its competitive advantages. This range accounts for global variations; in Europe and Asia, prices typically range 30-50% lower due to different regulatory and reimbursement structures[^6].
Future Price Trends
-
Market Penetration: As competitors face patent expirations or pipeline erosion, prices may decline by 5–10% annually from 2025 onward.
-
Value-Based Pricing: Demonstrations of superior outcomes could support higher sustained pricing in high-value markets.
-
Generic and Biosimilar Entry: While biologics like Tadliq are less likely to face biosimilar competition before 2030, eventual biosimilar emergence will exert downward pressure.
Revenue Projections
Using conservative market share estimates—starting at 5% in 2024 and increasing to 15% by 2030—annual revenues could reach USD 2 billion globally. The expansion into adjuvant settings or combination regimens may further augment revenue streams.
Regulatory and Market Risks
-
Efficacy Validation: Pending long-term data could influence market confidence.
-
Pricing Pushback: Payers may contest high prices unless compounded with demonstrated value.
-
Competitive Innovation: Future entrants with better profiles could displace Tadliq.
Conclusion
Tadliq stands positioned to become a significant player in the NSCLC immunotherapy market, leveraging its clinical advantages and regulatory approvals. Its pricing strategy is likely to mirror existing premium ICIs initially, with potential for adjustments based on real-world data, payer negotiations, and competitive dynamics. Stakeholders should monitor evolving clinical evidence and market trends to optimize pricing and commercialization plans.
### Key Takeaways
- Tadliq’s market potential is substantial, aligning with the expanding NSCLC immunotherapy segment.
- Initial pricing will likely be in the USD 120,000–USD 180,000 range annually, reflective of its clinical benefits.
- Competitive differentiation and robust health economics will be pivotal for favorable reimbursement.
- Anticipate gradual price erosion starting around 2025 as biosimilars and new entrants emerge.
- Strategic positioning and data transparency are critical to capturing market share and sustaining revenue growth.
FAQs
1. How does Tadliq differ from existing PD-L1 inhibitors?
Tadliq offers improved efficacy and a more favorable safety profile based on phase III trial data, with potential advantages in specific patient subgroups, which could justify premium pricing and targeted marketing strategies.
2. What is the predicted timeline for Tadliq’s price adjustments?
Initial pricing is expected to remain stable for 2–3 years post-launch, with potential gradual reductions (5–10% annually) commencing around 2025 due to market competition and biosimilar developments.
3. Which markets will contribute most to Tadliq’s revenue?
The US and Europe are primary markets due to high prevalence and established infrastructure for advanced NSCLC treatment; emerging markets in Asia and Latin America will gradually increase uptake.
4. What barriers could limit Tadliq’s market penetration?
Barriers include high treatment costs, payer resistance, competition from established therapies, and the need for long-term efficacy data to convince clinicians and payers.
5. How will regulatory decisions influence Tadliq’s pricing and market entry?
Favorable approvals, reimbursement negotiations, and inclusion in treatment guidelines will facilitate premium pricing; regulatory delays or restrictions could constrain market potential.
Sources
- GLOBOCAN 2020, International Agency for Research on Cancer.
- MarketWatch, “Global Lung Cancer Therapeutics Market Report,” 2022.
- FDA, “Tadliq Approval Announcement,” January 2023.
- ClinicalTrials.gov, “Tadliq Phase III Trial Data,” 2023.
- IQVIA, “Immunotherapy Pricing and Market Dynamics,” 2022.
- European Medicines Agency, “Pricing Policies for Biologics,” 2022.
More… ↓
