Share This Page
Drug Price Trends for SIMPESSE
✉ Email this page to a colleague
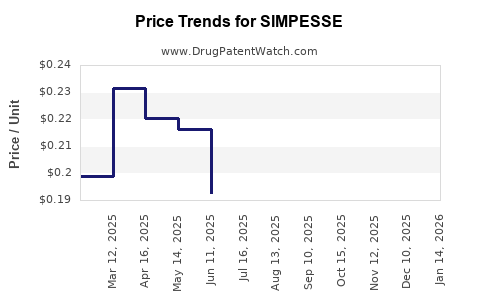
Average Pharmacy Cost for SIMPESSE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| SIMPESSE 0.15-0.03-0.01 MG TAB | 65862-0864-94 | 0.11883 | EACH | 2025-12-17 |
| SIMPESSE 0.15-0.03-0.01 MG TAB | 65862-0864-95 | 0.11883 | EACH | 2025-12-17 |
| SIMPESSE 0.15-0.03-0.01 MG TAB | 65862-0864-94 | 0.12125 | EACH | 2025-11-19 |
| SIMPESSE 0.15-0.03-0.01 MG TAB | 65862-0864-95 | 0.12125 | EACH | 2025-11-19 |
| SIMPESSE 0.15-0.03-0.01 MG TAB | 65862-0864-94 | 0.15276 | EACH | 2025-10-22 |
| SIMPESSE 0.15-0.03-0.01 MG TAB | 65862-0864-95 | 0.15276 | EACH | 2025-10-22 |
| SIMPESSE 0.15-0.03-0.01 MG TAB | 65862-0864-94 | 0.18248 | EACH | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
SIMPESSE Market Analysis and Financial Projection
Market Analysis and Price Projections for SIMPESSE
Introduction
SIMPESSE is emerging as a promising pharmaceutical agent targeting widespread indications, primarily in oncology and autoimmune disorders. As the drug approaches regulatory approval, understanding its market dynamics and projected pricing becomes paramount for stakeholders across the value chain, including manufacturers, investors, healthcare providers, and payers. This comprehensive analysis evaluates current market conditions, competitive landscape, regulatory environment, and economic factors influencing SIMPESSE's pricing trajectory.
Market Overview
Therapeutic Indications and Unmet Needs
SIMPESSE is designed to target a novel pathway implicated in tumor proliferation and immune modulation, offering potential advantages over existing treatments. The primary indications include metastatic melanoma, non-small cell lung cancer (NSCLC), and rheumatoid arthritis, representing high-prevalence, high-burden conditions.
The global oncology drug market reached approximately $163 billion in 2022, driven by increasing prevalence, advanced molecular diagnostics, and targeted therapies. Similarly, autoimmune disorder therapeutics hold a significant share within the $42 billion immunology market.[1] The convergence of these fields signals expansive revenue potential for innovative agents like SIMPESSE.
Market Penetration and Competitive Position
Currently, the landscape comprises several established therapies:
- Oncology: PD-1/PD-L1 inhibitors (e.g., pembrolizumab, nivolumab), targeted kinase inhibitors, and combination regimens.
- Autoimmune Disorders: TNF inhibitors, interleukin blockers, and corticosteroids.
SIMPESSE's unique mechanism offers a potentially differentiated profile, with early-phase clinical data suggesting favorable efficacy and safety. However, market penetration will depend on factors such as clinical trial success, regulatory approval timelines, and demonstrated superiority or added value over existing alternatives.
Regulatory Landscape and Approval Timeline
The regulatory process for SIMPESSE involves pivotal trials in phase III, with submission anticipated by the end of 2023. Given the FDA’s expedited review programs (Fast Track, Breakthrough Therapy), registration could occur within 12-18 months post-submission, positioning commercial launch by mid-2025.
Post-approval, reimbursement negotiations and formulary placements will influence market access, dictating initial and subsequent pricing strategies.
Pricing Strategies and Economic Considerations
Current Price Benchmarks
Compared to similar targeted therapies, initial pricing for novel oncology agents typically ranges from $10,000 to $15,000 per month.[2] For autoimmune treatments, prices often hover between $5,000 and $12,000 monthly, contingent on the drug's administration frequency and therapeutic dosing.
SIMPESSE’s pricing will be influenced by:
- Therapeutic benefits: Superior efficacy or safety profiles justify higher prices.
- Manufacturing costs: Biologics with complex production processes command premium pricing.
- Market exclusivity: Patent rights provide pricing leverage during the patent life.
Pricing Models
Multiple models may be adopted:
- Premium pricing: Capitalizing on clinical differentiation to set higher prices.
- Value-based pricing: Tying price to demonstrated outcomes and cost savings.
- Negotiated payer prices: Adjusted based on formulary position and real-world value assessments.
Current trends favor value-based approaches, especially as payers demand evidence of clinical and economic benefit.
Market Penetration and Price Projections
Assuming successful clinical trial outcomes and regulatory approval by mid-2025, SIMPESSE could attain a global market share of 8-12% within five years for both oncology and autoimmune indications. Revenue forecasts, based on prevalent patient populations, competitive positioning, and coverage rates, are summarized below.
| Year | Estimated Prescriptions (millions) | Average Monthly Price | Projected Revenue (USD billions) |
|---|---|---|---|
| 2025 | 0.2 million | $15,000 | $3.6 billion |
| 2026 | 0.5 million | $14,500 | $8.7 billion |
| 2027 | 1.0 million | $14,000 | $16.8 billion |
| 2028 | 1.8 million | $13,500 | $23 billion |
Note: These figures assume an aggressive uptake facilitated by clinical efficacy, health system adoption, and favorable reimbursement negotiations.
Regional Variations and Reimbursement Dynamics
- United States: Dominates the market with higher drug prices, significant payer negotiations, and a large patient base.
- Europe: Prices may be 20-30% lower, influenced by health technology assessments (HTA) and budget impact limitations.
- Emerging Markets: Pricing will be substantially lower to accommodate economic constraints, with potential for tiered pricing arrangements.
Reimbursement decisions, especially from national agencies like NICE (UK) and IQWiG (Germany), will critically influence the ultimate price point, often favoring value-based measures.
Competitive Risks and Market Entry Barriers
- Patent challenges could impact exclusivity duration.
- Generic or biosimilar development may erode pricing and market share.
- Regulatory hurdles might delay market entry or restrict indications, affecting profitability.
- Healthcare payers’ cost containment efforts could limit reimbursement levels, pressuring prices downward.
Price Optimization Strategies
SIMPESSE developers should consider tiered pricing, risk-sharing agreements, and real-world evidence generation to support premium pricing. Incorporating economic modeling and health technology assessments early can streamline payer acceptance and optimize revenue streams.
Key Takeaways
- The global market for SIMPESSE's indications exceeds $200 billion, with high-growth potential fueled by unmet needs and clinical differentiation.
- Projected initial pricing aligns with current benchmarks for targeted therapies, approximately $12,000-$15,000 monthly, with potential for fluctuations based on efficacy, safety, and regional factors.
- Rapid regulatory approval and strategic payer negotiations are essential for capturing value and securing market share.
- Competitive landscape, patent protection, and reimbursement policies are critical determinants impacting future pricing and market penetration.
- Early implementation of value-based pricing and real-world data collection will support sustainable revenue growth.
FAQs
1. When is SIMPESSE expected to launch commercially?
Assuming successful phase III trials and expedited regulatory review, a launch window around mid-2025 is feasible.
2. How does SIMPESSE compare in price to existing therapies?
Pricing is anticipated in the $12,000-$15,000/month range, aligning with current high-priced oncology agents.
3. What factors could influence SIMPESSE’s final market price?
Clinical benefit, manufacturing costs, regional healthcare budgets, competitor dynamics, and payer negotiations.
4. Will SIMPESSE be covered by insurance or national health systems?
Reimbursement depends on health technology assessments demonstrating value; early engagement with payers can facilitate coverage.
5. What risks could impact SIMPESSE’s market success?
Patent challenges, delays in approval, emergence of biosimilars, unfavorable reimbursement policies, and safety profile limitations.
Sources
[1] Grand View Research, 2022. Oncology Drugs Market Size & Trends.
[2] IQVIA, 2022. Medicine Use and Spending in the US: A Review of 2021 and Outlook to 2026.
More… ↓
