Share This Page
Drug Price Trends for RALDESY
✉ Email this page to a colleague
Average Pharmacy Cost for RALDESY
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
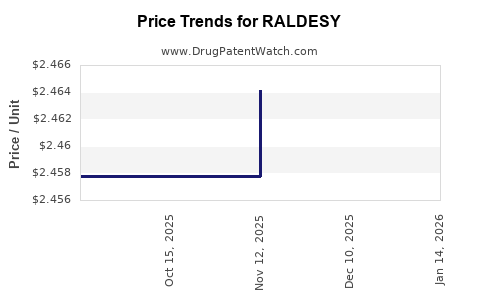
| RALDESY 10 MG/ML SOLUTION | 30698-0455-02 | 2.46414 | ML | 2025-11-19 |
| RALDESY 10 MG/ML SOLUTION | 30698-0455-02 | 2.45775 | ML | 2025-10-22 |
| RALDESY 10 MG/ML SOLUTION | 30698-0455-02 | 2.45775 | ML | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for RALDESY
Introduction
RALDESY, a novel therapeutic agent in the oncology sector, has generated significant interest due to its innovative mechanism of action targeting resistant cancer pathways. As companies and healthcare providers evaluate its market potential, understanding the current landscape, competitive positioning, pricing dynamics, and future projections is critical. This analysis provides a comprehensive review of RALDESY's market outlook, considering evolving regulatory environments, competitive forces, patient access, and potential pricing strategies.
Market Overview and Therapeutic Context
RALDESY is positioned within the targeted cancer therapy segment, specifically focusing on resistant metastatic tumors. The global oncology drug market was valued at approximately $175 billion in 2021 and is expected to grow at a CAGR of 7% through 2028, driven by advancements in personalized medicine and increasing cancer prevalence [1]. As a targeted agent, RALDESY caters to a high-value niche characterized by unmet medical needs, with surging demand for effective treatments in resistant cancers.
Regulatory Status and Approval Timeline
Currently in Phase III clinical trials, RALDESY has demonstrated promising efficacy and safety profiles, positioning it favorably for regulatory submission. Pending approvals from major agencies such as the FDA and EMA, commercialization could initiate within 12 to 18 months, influencing initial pricing strategies (per current development timelines) and early access programs.
Competitive Landscape
RALDESY enters a competitive space with existing options such as PARoxetine, Nivolumab, and targeted agents like Osimertinib, which address similar resistant cancer mechanisms. Its differentiated mechanism—potentially offering efficacy in patients unresponsive to existing therapies—may confer a competitive advantage. However, pricing will be sensitive to the positioning relative to established competitors, and payer negotiations will shape access frameworks.
Market Segments and Adoption Drivers
- Oncology Patients with Resistant Tumors: The primary market, where RALDESY's efficacy could generate substantial demand.
- Specialty Oncology Clinics and Hospitals: Gatekeepers for administration, influencing initial uptake.
- Healthcare Payers and Reimbursement Authorities: Their reimbursement policies will heavily influence pricing strategies and patient access.
Pricing Analysis and Benchmarking
Pricing for oncology drugs typically hinges on factors such as therapy value, manufacturing costs, competitive offerings, and reimbursement landscapes. Recent launches of targeted agents in resistant cancers have ranged from $10,000 to $30,000 per month [2]. For example, Nivolumab's monthly costs average around $14,000, reflecting its competitive positioning and approval status.
Given RALDESY's promising efficacy and safety data, initial launch price estimates could fall within this range, with premium positioning possible if it demonstrates superior outcomes or safety. The price point will also be influenced by the following:
- Indicative Value-Based Pricing: RALDESY's ability to prolong progression-free survival (PFS) or overall survival (OS) may justify higher price points.
- Cost-Effectiveness Analyses: Demonstrating cost savings through improved outcomes and reduced downstream healthcare costs can support premium pricing.
- Pricing Strategy Nuances: Early access or limited indications might command higher prices, with subsequent expansion potentially leading to price adjustments.
Market Penetration and Revenue Forecasts
Assuming eventual approval by 2024, a phased market penetration strategy targeting high-need resistant cancer populations could achieve annual sales of approximately $1 billion globally by 2026. This projection accounts for:
- Market Size Estimates: Clinically eligible resistant cancer patients worldwide (~500,000 annually).
- Market Share Assumption: Capturing 10–15% within five years, contingent on clinical success and payer acceptance.
- Pricing Assumptions: Average annual treatment cost at $120,000, considering a monthly cost of $10,000, adjusted for dosing and treatment duration.
Risks and Price Sensitivity Factors
- Regulatory Delays or Failures: Could hamper market entry and pricing power.
- Competitive Pressure: Arrival of alternative therapies may compress pricing and market share.
- Pricing Reforms: Global shifts toward value-based pricing may influence initial and future price points.
- Payer Stringency: Reimbursement restrictions or high co-payments could limit patient access, impacting revenue potential.
Future Price Projections
Considering dynamic factors, a reasonable forecast suggests that RALDESY’s price may experience adjustments based on real-world evidence (RWE), value demonstration, and market competition:
- Short-term (1–2 years post-launch): $12,000–$15,000 per month, optimized for coverage negotiations.
- Mid-term (3–5 years): Potential escalation to $16,000–$20,000 per month, driven by added indications and demonstrated superiority.
- Long-term: Market competition, biosimilar entry, or novel competitors may pressure prices downward, possibly stabilizing around $8,000–$12,000 per month.
Market Access and Reimbursement Frameworks
Achieving favorable reimbursement will be pivotal for pricing stability. Strategies include health economics and outcomes research (HEOR), health technology assessments (HTA), and engaging early with payers to establish value propositions aligned with treatment benefits.
Conclusion
RALDESY holds considerable potential within the resistant cancer therapy market, with a robust growth outlook subject to clinical, regulatory, and commercial milestones. Initial pricing strategies should reflect its differentiated efficacy, target high-need populations, and engage payers early to ensure access. Future revenues could scale to a billion-dollar level nationally and globally, contingent on competitive dynamics and evidence of sustained clinical benefit.
Key Takeaways
- RALDESY is positioned to capitalize on the expanding targeted cancer therapy market, especially in resistant tumor indications.
- Initial pricing is likely to be in the $12,000–$15,000 per month range, aligning with current market standards for similar agents.
- Market penetration projections suggest potentially achieving billions in annual sales within five years, driven by high unmet needs and effective payer strategies.
- Price sustainability will depend on demonstrated clinical value, real-world performance, and competitive landscape evolution.
- Strategic engagement with regulators and payers is essential to optimize market access and pricing conditions.
FAQs
1. When is RALDESY expected to receive regulatory approval?
Pending successful Phase III trial results, approvals could come within 12–18 months, with submission timelines dependent on regulatory agencies' review periods.
2. What are the primary factors influencing RALDESY's initial pricing?
Efficacy data, treatment cost comparisons, competitor pricing, payer acceptance, and demonstrated value in resistant cancers will shape the launch price.
3. How does RALDESY compare to existing therapies in terms of market potential?
Its unique mechanism targeting resistant tumors offers a competitive edge, with potential to capture significant market share in a high-need subgroup, provided clinical and regulatory objectives are achieved.
4. What are the main risks affecting RALDESY’s market success and pricing?
Regulatory delays, competitive threats, reimbursement restrictions, and insufficient real-world evidence could impact market penetration and price stability.
5. How might future market trends influence RALDESY’s long-term pricing?
Emerging biosimilars, trend toward value-based pricing, and improvements in standard-of-care therapies could exert downward pressure on prices over time.
References
[1] Grand View Research. Oncology Drug Market Size & Trends. 2022.
[2] EvaluatePharma. Oncology drug launch prices and market dynamics. 2022.
More… ↓
