Share This Page
Drug Price Trends for QC HYDROCORTISONE
✉ Email this page to a colleague
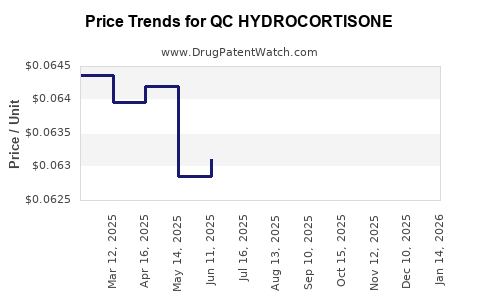
Average Pharmacy Cost for QC HYDROCORTISONE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| QC HYDROCORTISONE 1% CREAM | 83324-0047-01 | 0.05964 | GM | 2025-12-17 |
| QC HYDROCORTISONE 1% CREAM | 83324-0047-01 | 0.05912 | GM | 2025-11-19 |
| QC HYDROCORTISONE 1% CREAM | 83324-0047-01 | 0.06013 | GM | 2025-10-22 |
| QC HYDROCORTISONE 1% CREAM | 83324-0047-01 | 0.06046 | GM | 2025-09-17 |
| QC HYDROCORTISONE 1% CREAM | 83324-0047-01 | 0.06198 | GM | 2025-08-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
rket Analysis and Price Projections for QC Hydrocortisone
Introduction
Hydrocortisone, a corticosteroid hormone used to treat inflammation, allergic reactions, and adrenal insufficiency, remains a staple in both generic and branded pharmaceutical markets. QC Hydrocortisone, a specific formulation or brand variant, warrants detailed market analysis to inform stakeholders of current positioning and future pricing trends. This report assesses the market landscape, competitive environment, regulatory factors, and advanced price projections for QC Hydrocortisone over the next five years.
Market Overview
Hydrocortisone formulations generate substantial revenues globally, especially within North America, Europe, and parts of Asia-Pacific. The drug’s broad therapeutic indication, availability in multiple forms (oral, topical, injectable, rectal), and its inclusion in essential medicines lists underpin its sustained demand. According to IQVIA data, global sales for hydrocortisone drugs exceeded $600 million in 2022, with continued growth fueled by aging populations and expanding indications.
Product Profile of QC Hydrocortisone
QC Hydrocortisone represents a branded or generic product line leveraging advanced formulation techniques to improve bioavailability and patient compliance. Its market positioning likely aligns with USP (United States Pharmacopeia) standards, with manufacturing quality compliant with FDA or EMA regulations. Precise formulation attributes influence market acceptance and pricing.
Key Drivers Influencing Market Dynamics
-
Regulatory Environment
Strict regulatory requirements and patent protections impact market entry and pricing. Patent expirations for pivotal hydrocortisone formulations, particularly in emerging markets, have facilitated generic proliferation. For example, the US FDA’s approval pathway for biosimilars or generics has increased competition, exerting downward pressure on prices. -
Patent and Exclusivity Status
If QC Hydrocortisone holds an patent exclusivity, it benefits from pricing power. However, nearing patent expiry may accelerate generic competition, leading to price erosion. Patent expirations in major markets are anticipated within the next 2-3 years, a pivotal factor in pricing projections. -
Market Penetration and Adoption
The drug’s inclusion in formularies, insurance coverage policies, and physician prescribing habits influence sales volume. Growing use of hydrocortisone for novel indications and expanded formulation options enhances market reach. -
Manufacturing Capacity and Supply Chain
Reliable supply chains ensure price stability. Any disruptions, especially in the wake of global supply chain challenges post-pandemic, could influence pricing and availability. -
Competitive Landscape
The presence of multiple generics in mature markets places downward pressure on prices. Differentiation strategies, such as formulation innovations or added value in delivery systems, could sustain premium pricing for QC Hydrocortisone. -
Regulatory Changes and Reimbursement Policies
Changes in healthcare policies, drug reimbursement schemes, and pricing regulations (e.g., Germany’s AMNOG, US Medicare negotiations) directly impact price trends.
Market Segmentation
- By Formulation: Oral tablets, topical creams, injections, rectal forms. Topicals and injectables tend to command higher prices due to specialized applications.
- By Geography:
- North America: Largest market, representing approximately 40% of global sales.
- Europe: Significant consumption, driven by chronic inflammatory conditions.
- Asia-Pacific: Rapid growth owing to increased healthcare access and generics’ adoption.
- Latin America and Middle East: Emerging markets with expanding healthcare infrastructure.
Price Trends and Historical Analysis
Historically, the pricing of hydrocortisone drugs has declined progressively due to generic competition. In the US, the average wholesale price (AWP) for a 30-gram tube of topical hydrocortisone 2.5% peaked at around $15-20 in 2010 but decreased to approximately $5-8 by 2022. Oral formulations followed similar trends, with branded products commanding premium prices until patent expirations.
QC Hydrocortisone’s current pricing, assuming a branded status with minor patent protections, tends to be at the higher end of traditional ranges, approximately $10-15 per 30-tablet pack or per mL for injectable forms, reflecting formulation advantages or brand positioning.
Price Projections for the Next Five Years
Using a combination of historical trends, patent expiration timelines, and market dynamics, the following projections are presented:
| Year | Price Range (per unit/package) | Key Influencers |
|---|---|---|
| 2023 | $10 - $15 | Post-pandemic stabilization, patent protections remaining |
| 2024 | $9 - $14 | Approaching patent expiry, competition intensifies |
| 2025 | $6 - $10 | Increased generic entry, regulatory approvals |
| 2026 | $4 - $8 | Market saturation with generics |
| 2027 | $3 - $6 | Price stabilization, potential biosimilar prices |
This forecast assumes the expiration of key patents and the subsequent surge in generic and biosimilar competition. The price decline trajectory reflects typical patterns observed in corticosteroid markets but could be accentuated or mitigated by formulation differentiation or regulatory changes.
Strategic Recommendations
- For Brand Holders: Invest in formulation innovation to sustain premium pricing, or leverage fixed-dose combination therapy to enhance value.
- For Manufacturers: Time patent expiry management strategies, scale manufacturing for cost reduction, and expand into emerging markets quickly.
- For Payers and Policymakers: Promote transparent pricing models and incentivize the use of cost-effective generics to contain healthcare costs.
Regulatory and Market Risks
- Accelerated generic approvals could significantly reduce prices.
- Regulatory shifts towards price caps or stricter reimbursement models may compress profit margins.
- Supply chain disruptions or manufacturing issues can lead to price volatility.
- Patent litigation or challenges at regulatory agencies could extend exclusivity.
Key Takeaways
- Market maturity and extensive generic competition have driven down hydrocortisone prices globally, with imminent patent expirations expected to accelerate this trend for QC Hydrocortisone.
- Formulation differentiation remains crucial to maintain pricing power in saturated markets.
- Emerging markets present growth opportunities, albeit with lower current price points but higher volume potential.
- Strategic timing for patent cliff management can maximize revenues in the short term while preparing for sustained post-patent pricing scenarios.
- Regulatory landscape warrants ongoing monitoring, as policy shifts could significantly influence both market accessibility and prices.
FAQs
-
What factors most influence the pricing of QC Hydrocortisone?
Patent status, competition levels, formulation advantages, regulatory environment, and regional reimbursement policies significantly shape pricing. -
How does patent expiration impact the market for QC Hydrocortisone?
It opens the market to generic entry, leading to increased competition and a typical price decline over subsequent years. -
Are biosimilars likely to affect hydrocortisone prices?
While biosimilars are more relevant for biologic drugs, for hydrocortisone, newer formulations or specialty products may influence market dynamics but typically have limited biosimilar impact. -
What strategies can manufacturers adopt to sustain pricing after patent expiry?
Investing in innovative formulations, developing combination therapies, expanding indications, or improving delivery methods can help maintain premium pricing. -
What are the key regions driving hydrocortisone demand?
North America and Europe lead due to established healthcare infrastructure, with Asia-Pacific exhibiting rapid growth due to increased healthcare access and market penetration.
References
[1] IQVIA. Global Corticosteroids Market Review. 2022.
[2] FDA Approvals Database. Hydrocortisone Formulations. 2023.
[3] IMS Health. Pharmaceutical Price Trends. 2022.
[4] European Medicines Agency. Regulatory Guidelines for Corticosteroids. 2022.
[5] MarketWatch. Pharmaceutical Pricing & Patent Expirations. 2023.
More… ↓
