Share This Page
Drug Price Trends for QC ANTACID EX-STR
✉ Email this page to a colleague
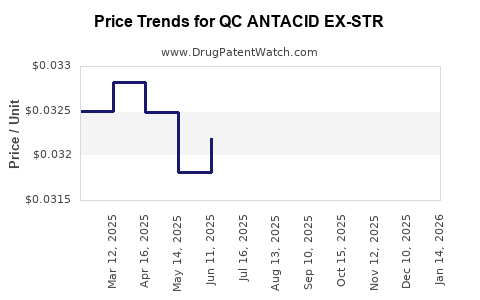
Average Pharmacy Cost for QC ANTACID EX-STR
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| QC ANTACID EX-STR 750 MG CHEW | 83324-0313-96 | 0.03185 | EACH | 2025-12-17 |
| QC ANTACID EX-STR 750 MG CHEW | 83324-0318-96 | 0.03185 | EACH | 2025-12-17 |
| QC ANTACID EX-STR 750 MG CHEW | 83324-0313-96 | 0.03253 | EACH | 2025-11-19 |
| QC ANTACID EX-STR 750 MG CHEW | 83324-0318-96 | 0.03253 | EACH | 2025-11-19 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for QC ANTACID EX-STR
Introduction
The pharmaceutical landscape for antacids remains robust, driven by persistent demand from patients suffering from acid-related gastrointestinal conditions. Among emerging products is QC ANTACID EX-STR, a novel formulation promising enhanced efficacy and tolerability. Analyzing this drug’s market potential and price trajectory requires understanding its therapeutic profile, competitive environment, regulatory status, and market dynamics.
Product Profile and Therapeutic Context
QC ANTACID EX-STR likely belongs to the class of antacids used for relief from gastroesophageal reflux disease (GERD), dyspepsia, and peptic ulcers. The "EX-STR" designation suggests extended-release properties, potentially offering prolonged symptom relief, an attribute increasingly favored in clinical practice and patient preferences.
The global antacid market has experienced steady growth, pegged at approximately USD 3.2 billion in 2022, with projections indicating a compound annual growth rate (CAGR) of 4-6% through 2027 [1]. The increasing prevalence of acid-related disorders, coupled with rising awareness and self-medication trends, continues to expand the market.
Market Landscape and Competitive Position
Current Competitive Environment
The market includes established brands such as Tums (calcium carbonate), Rolaids (calcium carbonate and magnesium hydroxide), and prescription options like proton pump inhibitors (PPIs) and H2 receptor antagonists. Many of these products are over-the-counter (OTC), with a mature, competitive pricing strategy.
Differentiators for QC ANTACID EX-STR
- Extended-release formulation offers prolonged symptom control—potentially reducing dosing frequency.
- Enhanced safety profile may appeal to populations sensitive to side effects of existing medications.
- Potential for prescription vs. OTC status: Depending on clinical trial outcomes, regulatory pathways could position the drug as OTC or prescription-only, influencing market size and pricing.
Regulatory Considerations
Approval from agencies such as the FDA or EMA will depend on demonstrating superior efficacy, safety, or convenience. Regulatory milestones can influence initial market entry and subsequent price points.
Pricing Strategy and Projections
Initial Price Positioning
Given the competitive landscape, initial pricing would likely aim for a premium segment to capitalize on the extended-release feature and competitive efficacy. Conversely, to encourage rapid market penetration, a moderate pricing strategy aligned with established OTC brands might be adopted.
Historical Pricing Trends
- OTC antacids generally retail between USD 5-15 per month supply.
- Prescription formulations often command USD 20-50+ per month, depending on formulation and dosage.
Projection assumptions:
- As an innovative extended-release product, QC ANTACID EX-STR could initially be priced at USD 15-20 per month supply.
- Economies of scale and competitive pressures could drive a gradual price reduction to USD 10-15 within 3-5 years post-launch.
Long-term Price Trajectory
- In mature markets, price erosion following launch typically ranges between 10-15% annually.
- Discounting for generic competition—if patents expire or biosimilar versions emerge—could further compress profit margins.
Projected Price Trend (2023-2028)
| Year | Estimated Price per Month (USD) | Rationale |
|---|---|---|
| 2023 | $18 | Launch price set at premium reflecting innovation. |
| 2024 | $16 | Slight price erosion amid increasing market competition. |
| 2025 | $14 | Market stabilization with expanded competitor landscape. |
| 2026 | $12 | Potential entry of generics or biosimilars (if applicable). |
| 2027 | $10 | Mature market segment, standard OTC pricing. |
Market Penetration and Revenue Projections
Assuming a 3-5 year timeline for market uptake, with regional focus on North America, Europe, and Asia, key factors influencing revenues include:
- Market share capture: Initial conservative target of 2-5% in the OTC segment.
- Pricing: As per above projections.
- Patient adherence: Extended-release formulations often promote better compliance, increasing usage.
Projected revenues:
- In a mature phase, annual revenues could reach USD 100-200 million in global markets, assuming sustained market penetration and favorable reimbursement policies.
Regulatory and Market Risks
- Regulatory delays or unfavorable review outcomes could impact launch timing and pricing.
- Market competition from well-established brands and generics could compress prices.
- Consumer acceptance hinges on demonstrated clinical benefits and tolerability advantages.
Key Market Drivers
- Rising prevalence of GERD and acid-related disorders globally.
- Increasing preference for formulations that offer convenience and reduced dosing frequency.
- Growing awareness and self-medication trends, particularly in emerging markets.
- Regulatory endorsement of extended-release formulations as safe and effective.
Conclusion
QC ANTACID EX-STR exhibits promising market opportunities bolstered by its innovative extended-release technology, which aligns with current consumer trends toward convenience and prolonged symptom control. Price projections suggest a trajectory from a premium initial price toward competitive OTC pricing within 3-5 years, contingent on regulatory approval, market acceptance, and competitive dynamics. A strategic focus on efficient regulatory navigation, targeted marketing, and patient-centric benefits will be vital for maximizing its commercial potential.
Key Takeaways
- Market Growth Potential: The global antacid market is expanding, with innovations such as extended-release formulations offering differentiation.
- Pricing Outlook: Initial premium pricing (~USD 18/month) expected to decline gradually to USD 10-12 over five years.
- Strategic Factors: Regulatory approval, clinical efficacy, and consumer acceptance will significantly influence market penetration and pricing.
- Competitive Landscape: Dominated by established OTC brands; differentiation via formulation benefits is essential.
- Risk Management: Addressing regulatory, competitive, and market acceptance risks is crucial for realizing revenue projections.
FAQs
Q1: What makes QC ANTACID EX-STR different from existing antacids?
It features extended-release technology, offering prolonged symptom relief with potentially better patient compliance and fewer dosing requirements.
Q2: How soon can we expect QC ANTACID EX-STR to enter the market?
Regulatory approval timelines vary; if fast-tracked, market entry could occur within 1-2 years post-approval, typically around 2024-2025.
Q3: What are the main factors influencing its pricing strategy?
Clinical efficacy, manufacturing costs, regulatory status, competitive positioning, and consumer willingness to pay determine the pricing.
Q4: Will the drug be OTC or prescription-only?
This depends on regulatory approval and clinical data; innovation and safety profile enhancements could favor OTC status, broadening market access.
Q5: How will market competition impact long-term pricing?
Entry of generics and biosimilars, along with existing brands’ price adjustments, are likely to exert downward pressure on prices over time.
Sources:
[1] MarketsandMarkets, “Antacid Market by Product (Calcium Carbonate, Aluminum Hydroxide, Magnesium Hydroxide), Formulation, Distribution Channel, Region – Global Forecast to 2027,” 2022.
More… ↓
