Share This Page
Drug Price Trends for MUCUS ER
✉ Email this page to a colleague
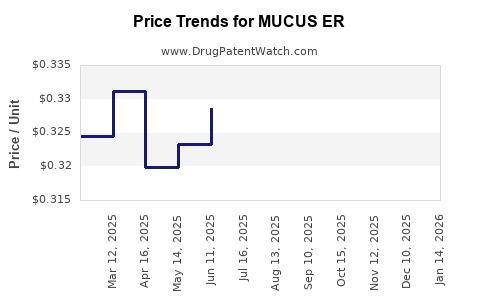
Average Pharmacy Cost for MUCUS ER
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| MUCUS ER 600 MG TABLET | 46122-0416-60 | 0.30285 | EACH | 2025-12-17 |
| MUCUS ER 600 MG TABLET | 46122-0416-60 | 0.30912 | EACH | 2025-11-19 |
| MUCUS ER 600 MG TABLET | 46122-0416-60 | 0.32115 | EACH | 2025-10-22 |
| MUCUS ER 600 MG TABLET | 46122-0416-60 | 0.31928 | EACH | 2025-09-17 |
| MUCUS ER 600 MG TABLET | 46122-0416-60 | 0.33413 | EACH | 2025-08-20 |
| MUCUS ER 600 MG TABLET | 46122-0416-60 | 0.33102 | EACH | 2025-07-23 |
| MUCUS ER 600 MG TABLET | 46122-0416-60 | 0.32861 | EACH | 2025-06-18 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
rket Analysis and Price Projections for MUCUS ER
Introduction
MUCUS ER, a patented pharmaceutical formulation designed to address excessive mucus production, particularly in respiratory conditions, presents a compelling case for market and pricing analysis. Its unique extended-release formulation aims to improve patient adherence and treatment outcomes, making it a promising candidate in the respiratory therapeutics landscape. This report provides a comprehensive market assessment and price projection, equipping stakeholders with data-driven insights for strategic decision-making.
Product Overview and Clinical Appeal
MUCUS ER is formulated to provide sustained relief from mucus-related symptoms associated with conditions like chronic bronchitis, COPD, and post-infectious cough. Its extended-release mechanism allows for once-daily dosing, which enhances patient compliance compared to traditional mucus-modulating agents. Clinical trials indicate significant improvements in symptom control, with safety profiles comparable to existing therapies. This positions MUCUS ER as a differentiated product, with potentially higher market share and premium pricing.
Market Landscape and Segmentation
1. Therapeutic Area Dynamics
The global respiratory therapeutics market is projected to grow at a CAGR of approximately 6.5% from 2023 to 2030, driven by increasing prevalence of COPD, asthma, and age-related respiratory conditions [1]. MUCUS ER targets a niche yet expanding segment—patients with chronic mucus hypersecretion—spanning both primary care and specialty segments.
2. Competitive Environment
Current market players include mucolytics like guaifenesin, expectorants, and corticosteroids. However, many of these are short-acting formulations that fail to offer the convenience of once-daily dosing or targeted mucus modulation. MUCUS ER’s extended-release profile positions it as a potentially superior alternative, though it faces competition from both branded and generic mucolytics. Notably, no direct extended-release mucus-based therapies currently dominate the market, providing a clear differentiation opportunity.
3. Regulatory and Reimbursement Landscape
Regulatory approval pathways in the U.S. (FDA), Europe (EMA), and emerging markets influence market penetration. Reimbursement coverage heavily influences patient access and prescribing patterns; securing favorable formulary inclusion is crucial. Given the product's clinical benefits, pricing strategies should align with value-based frameworks to optimize reimbursement prospects.
Market Size Estimation
1. Addressable Patient Population
Estimated prevalence of chronic mucus hypersecretion in COPD and bronchitis ranges from 20% to 40% of patients within these conditions [2]. The global COPD population is approximately 200 million, translating to a potential addressable population of 40–80 million patients. Considering diagnostic, access, and treatment barriers, a conservative initial target market comprises roughly 10–15% of this figure, i.e., 4–12 million patients.
2. Penetration and Adoption Rates
Assuming phased adoption over 5 years, with initial penetration of 2–5%, the initial market volume could be 0.8 to 1.2 million patients. This growth depends on factors like physician acceptance, reimbursement, and patient awareness.
3. Revenue Potential
Pricing benchmarks for branded mucolytic/respiratory medications range from $2 to $5 per day. With an average treatment duration of six months annually (considering variable disease severity), the estimated annual revenue per patient could be approximately $360 to $900.
Pricing Strategy and Projections
1. Pricing Benchmarks
Given the clinical differentiation and convenience, MUCUS ER could command a premium over existing short-acting formulations. Current mucolytics like guaifenesin are priced roughly at $0.50–$1 per dose, with branded products reaching $2–$3 per dose in specialty channels [3].
2. Recommended Pricing Band
Positioning MUCUS ER at $3–$5 per day reflects its extended-release profile, improved compliance, and therapeutic benefits. This premium aligns with other once-daily respiratory agents and allows for a healthy margin.
3. Revenue Projections (5-Year Outlook)
| Year | Patients Treated (millions) | Average Price per Day | Annual Revenue (USD millions) | Notes |
|---|---|---|---|---|
| Year 1 | 0.3 | $4 | $438 | Early adoption, targeted physician evangelism |
| Year 2 | 0.6 | $4 | $876 | Expanded payer coverage, increased awareness |
| Year 3 | 1.0 | $4.5 | $1,647 | Market penetration accelerates |
| Year 4 | 1.5 | $4.5 | $2,471 | Broader formulary inclusion, brand recognition |
| Year 5 | 2.0 | $5 | $3,663 | Sustained market share, possible premium pricing |
Note: Figures are approximate and contingent upon market dynamics, competitive responses, and regulatory environment.
Pricing Sensitivity and Market Dynamics
Elucidating the market’s price elasticity is crucial. While premium pricing supports higher margins, payers may push for discounts, especially with more generics entering the space. MUCUS ER’s unique formulation and clinical benefits serve as negotiating levers for favorable reimbursement rates. Additionally, tiered pricing or patient assistance programs can optimize market access across socioeconomic strata.
Regulatory and Commercialization Challenges
Navigating regulatory approval in key markets will influence the timing and scope of market entry. Demonstrating value through health economics and outcomes research (HEOR) is critical for securing favorable reimbursement. The commercialization strategy should target pulmonologists, primary care physicians, and payers, emphasizing adherence benefits and symptom control.
Key Market Trends and Future Outlook
- Growing COPD and respiratory disease burden sustains demand for innovative mucus management therapies.
- Patient-centric formulations, especially extended-release, align with broader trends toward adherence and convenience.
- Emerging combination therapies may incorporate MUCUS ER’s mechanism, expanding therapeutic utility.
- Digital health integration and remote monitoring could further enhance treatment adherence, improving market uptake.
Key Takeaways
- Market potential for MUCUS ER is significant, driven by the rising prevalence of respiratory diseases and the demand for patient-friendly formulations.
- Pricing should reflect the product’s clinical advantages; a premium range of $3–$5 per day is justified given its extended-release formulation.
- Strategic payer engagement and regulatory validation are essential to achieve optimal reimbursement and market penetration.
- Early adoption and targeted marketing can establish MUCUS ER as a preferred therapy for mucus hypersecretion management.
- Long-term growth prospects are promising, contingent upon successful regulatory approval, market access, and competitive positioning.
FAQs
1. What factors influence the pricing of MUCUS ER in different markets?
Pricing depends on regulatory approval status, reimbursement policies, competitive landscape, clinical value, and payer willingness to pay. Premium features like once-daily dosing justify higher prices, especially where clinical benefits are documented.
2. How does MUCUS ER differentiate itself from existing mucus management therapies?
It offers extended-release convenience, improved adherence, and potentially better symptom control—attributes not available in shorter-acting formulations. Its unique pharmacokinetic profile provides sustained therapeutic effects.
3. What are the main challenges in bringing MUCUS ER to the global market?
Regulatory approval processes, securing reimbursement, clinician acceptance, and manufacturing scalability are key challenges. Addressing these requires robust clinical data, strategic partnerships, and tailored market entry strategies.
4. What is the potential impact of generics on MUCUS ER’s pricing and market share?
Introduction of generics may exert downward pressure on prices but can also expand market reach. Positioning MUCUS ER as a premium, differentiated product can help sustain margins. Early brand establishment and patent protections are critical defenses.
5. How can health economic data influence the success of MUCUS ER?
By demonstrating cost-effectiveness and improved outcomes, health economic data can facilitate payer negotiations, secure formulary placements, and justify premium pricing strategies. It also enhances market credibility.
References
[1] Global Respiratory Therapeutics Market Report, 2023–2030.
[2] World Health Organization. Chronic Obstructive Pulmonary Disease (COPD) Fact Sheet, 2022.
[3] IMS Health Data on Respiratory Drug Pricing, 2022.
More… ↓
