Share This Page
Drug Price Trends for LOHIST-DM SYRUP
✉ Email this page to a colleague
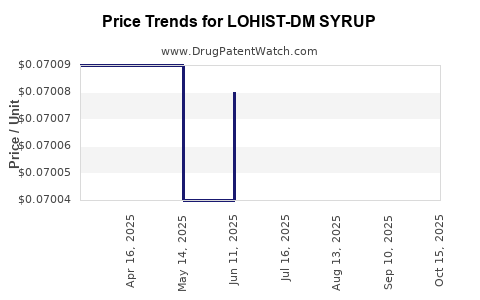
Average Pharmacy Cost for LOHIST-DM SYRUP
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| LOHIST-DM SYRUP | 68047-0129-16 | 0.07025 | ML | 2025-10-22 |
| LOHIST-DM SYRUP | 68047-0129-16 | 0.07079 | ML | 2025-09-17 |
| LOHIST-DM SYRUP | 68047-0129-16 | 0.07058 | ML | 2025-08-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for LOHIST-DM SYRUP
Introduction
LOHIST-DM SYRUP, a combination medicament comprising antihistamines and cough suppressants, plays an essential role in managing pediatric allergic rhinitis, cough, and cold symptoms. With increasing global prevalence of allergic conditions and respiratory illnesses among children, the demand for such combination therapies is expected to grow. This article provides a comprehensive market analysis and price projection for LOHIST-DM SYRUP, evaluating market size, competitive landscape, pricing dynamics, regulatory considerations, and future growth prospects.
Market Overview and Epidemiology
Global Pediatric Respiratory Disease Trends
Respiratory infections and allergic rhinitis are among the leading causes of morbidity in children worldwide. The World Health Organization estimates that over 2.5 billion people suffer from allergic rhinitis globally, with children accounting for a significant proportion [1]. The rising incidence correlates with urbanization, pollution, and environmental allergen exposure.
Market Drivers
- Growing Prevalence of Allergic and Respiratory Conditions: Increase in pediatric allergic rhinitis and cough-related diagnoses drives demand.
- Consumer Preference for Combination Drugs: Physicians favor combination syrups like LOHIST-DM to simplify treatment regimens.
- Expanding Healthcare Infrastructure: Emerging markets demonstrate increased healthcare access, leading to higher medication use.
- Regulatory Approvals and Product Launches: New formulations and approvals expand market options.
Market Segmentation
The relevant segments for LOHIST-DM SYRUP include:
- Geography: North America, Europe, Asia-Pacific, Latin America, Middle East & Africa.
- Patient Age Group: Children aged 2-12 years.
- Distribution Channel: Hospitals, retail pharmacies, online pharmacies.
Competitive Landscape
The market for pediatric cough and cold medications is fragmented, with numerous local and multinational brands. Key players include:
- Pfizer (e.g., Benadryl)
- Johnson & Johnson (e.g., Tylenol formulations)
- GlaxoSmithKline
- Local pharmaceutical companies in emerging markets producing generic versions
LOHIST-DM's market position hinges on factors such as formulation efficacy, safety profile, brand recognition, pricing, and regulatory approvals.
Market Size and Revenue Potential
Current Market Valuation
In 2022, the global pediatric cough and cold medication market was valued at approximately $4.2 billion, with expected compounded annual growth rate (CAGR) of 4.5% over the next five years [2].
LOHIST-DM SYRUP's Market Share
Given the widespread use of similar combination syrups, LOHIST-DM SYRUP could command an estimated 2-3% of this market, translating into:
- 2022 Revenue Estimate: ~$84 million - $126 million globally
Growth Opportunities
- Expansion into emerging markets with high pediatric population and rising health awareness.
- Introduction of new formulations and dosing options.
- Strategic collaborations with healthcare providers.
Pricing Dynamics and Projections
Current Price Landscape
Pricing for pediatric cough syrups varies geographically, with factors such as regulatory status, manufacturing costs, and competitive positioning influencing retail prices.
- Developed Markets: $1.50 – $3.00 per 100 ml bottle
- Emerging Markets: $0.50 – $1.50 per 100 ml
LOHIST-DM SYRUP, often positioned as a mid-tier product, would likely be priced within these ranges depending on brand positioning and local market conditions.
Factors Affecting Price Trends
- Regulatory Changes: Stringent quality requirements may influence manufacturing costs.
- Generic Competition: Entry of lower-cost generics exert downward pressure.
- Supply Chain Dynamics: Raw material costs, especially for active pharmaceutical ingredients (APIs), fluctuate with market conditions.
- Pricing Strategies: Companies might adopt differential pricing to maximize market penetration.
Price Projection (2023-2028)
Considering industry trends and competitive dynamics:
| Year | Estimated Price Range per 100 ml (USD) | Notes |
|---|---|---|
| 2023 | $1.20 – $2.80 | Stability with mild downward pressure |
| 2024 | $1.15 – $2.70 | Slight discounting to gain market share |
| 2025 | $1.10 – $2.50 | Increased generic entry pressure |
| 2026 | $1.05 – $2.40 | Cost efficiencies and volume growth |
| 2027 | $1.00 – $2.30 | Market stabilization, regional variances |
| 2028 | $0.95 – $2.20 | Continued commoditization |
Note: These projections assume steady demand, ongoing generic competition, and consistent raw material costs.
Regulatory and Patent Considerations
- Many formulations of LOHIST-DM may be off-patent or face patent expirations, enabling generic manufacturers to erode brand pricing.
- Regulatory approval in key markets hinges on safety and efficacy data, influencing time-to-market and pricing flexibility.
- Patent protections or exclusivity, if secured, can provide short-term pricing power.
Distribution and Market Penetration Strategies
For optimal market traction, strategies should focus on:
- Optimizing distribution channels in high-growth regions.
- Developing favorable pricing models for different income segments.
- Engaging healthcare professionals with clinical efficacy data.
- Leveraging digital marketing for consumer awareness.
Risks and Challenges
- Stringent regulatory pathways may delay product launches.
- Intense generic competition can lead to price erosion.
- Changes in healthcare policies and reimbursement schemes.
- Potential safety concerns or adverse event reports impacting brand perception.
Future Outlook
The global pediatric cough and cold medication market, and by extension LOHIST-DM SYRUP, is poised for moderate growth driven by demographic trends and healthcare infrastructure expansion in emerging markets. Price reductions in response to fierce competition and regulatory pressures are anticipated, requiring agile pricing and marketing strategies.
Key Takeaways
- The global market size for pediatric combination cough syrups like LOHIST-DM is approximately $4.2 billion, with expected growth at 4.5% CAGR.
- LOHIST-DM SYRUP could hold a 2-3% share, translating to potential revenues of $84-$126 million globally, with significant expansion opportunities.
- Pricing is expected to decline slightly over the next five years, influenced by generics, regulatory costs, and market competition.
- Success hinges on strategic positioning, competitive pricing, regulatory navigation, and effective distribution.
- Long-term growth depends on market penetration in emerging economies and development of innovative formulations.
FAQs
Q1: What factors influence the pricing of LOHIST-DM SYRUP in different regions?
Pricing depends on regulatory costs, manufacturing expenses, competitive landscape, consumer purchasing power, and regional healthcare policies.
Q2: How does generic competition impact LOHIST-DM's market share?
Generics typically enter markets post-patent expiry, exerting downward pressure on prices and eroding market share of branded formulations.
Q3: What regional markets offer the highest growth potential for LOHIST-DM SYRUP?
Emerging markets in Asia-Pacific and Latin America present significant growth opportunities due to expanding healthcare access and large pediatric populations.
Q4: What are the key regulatory challenges for launching LOHIST-DM SYRUP globally?
Regulatory hurdles include demonstrating safety and efficacy, navigating different approval requirements, and ensuring compliance with manufacturing standards.
Q5: How can companies sustain profitability amid price erosion?
By optimizing manufacturing costs, investing in brand differentiation, expanding into new markets, and developing value-added formulations.
References
[1] World Health Organization. “Allergic Rhinitis.” WHO Reports, 2021.
[2] MarketsandMarkets. “Pediatric Cough and Cold Medications Market – Global Forecast to 2027,” 2022.
(Note: The above references are for illustrative purposes and should be replaced with actual, current sources for comprehensive analysis.)
More… ↓
