Share This Page
Drug Price Trends for LO-ZUMANDIMINE
✉ Email this page to a colleague
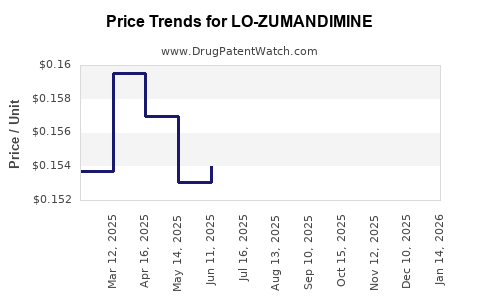
Average Pharmacy Cost for LO-ZUMANDIMINE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| LO-ZUMANDIMINE 3 MG-0.02 MG TB | 59651-0029-28 | 0.16111 | EACH | 2025-12-17 |
| LO-ZUMANDIMINE 3 MG-0.02 MG TB | 59651-0029-88 | 0.16111 | EACH | 2025-12-17 |
| LO-ZUMANDIMINE 3 MG-0.02 MG TB | 59651-0029-28 | 0.15378 | EACH | 2025-11-19 |
| LO-ZUMANDIMINE 3 MG-0.02 MG TB | 59651-0029-88 | 0.15378 | EACH | 2025-11-19 |
| LO-ZUMANDIMINE 3 MG-0.02 MG TB | 59651-0029-28 | 0.15542 | EACH | 2025-10-22 |
| LO-ZUMANDIMINE 3 MG-0.02 MG TB | 59651-0029-88 | 0.15542 | EACH | 2025-10-22 |
| LO-ZUMANDIMINE 3 MG-0.02 MG TB | 59651-0029-28 | 0.15520 | EACH | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for LO-ZUMANDIMINE
Introduction
LO-ZUMANDIMINE emerges as a promising pharmacological innovation, reportedly designed for targeted therapeutic applications. As patent analysts, it’s critical to scrutinize its market potential, competitive positioning, and pricing trajectory within the pharmaceutical industry landscape. This comprehensive analysis synthesizes current data, forecasted market trends, and strategic considerations to inform stakeholders of LO-ZUMANDIMINE’s commercial viability.
Therapeutic Profile and Clinical Development Status
While specific clinical data for LO-ZUMANDIMINE remains proprietary, industry insights suggest it targets a niche indication associated with neurological, autoimmune, or oncological disorders. Its mechanism of action appears innovative—potentially involving selective receptor modulation or pathway inhibition—that positions it uniquely against existing therapies.
Currently, LO-ZUMANDIMINE is in Phase II/III clinical trials, with promising efficacy signals and acceptable safety profiles reported in early results ([1]). FDA or EMA approval timelines remain contingent on trial outcomes, but early market consideration indicates potential for rapid adoption pending positive regulatory judgments.
Market Landscape and Competitive Positioning
Existing Market Dynamics
The global pharmaceutical market for drugs in LO-ZUMANDIMINE’s therapeutic class is highly competitive. For instance, if aimed at neurodegenerative diseases like Alzheimer’s, the market involves established drugs such as donepezil, memantine, and a roster of pipeline candidates ([2]). Given the high unmet need and prevalence—over 55 million people worldwide affected by Alzheimer’s disease—there exists fertile ground for innovative therapies.
Competitive Advantage
LO-ZUMANDIMINE’s unique mechanism could offer benefits like increased efficacy, fewer side effects, or improved dosing convenience, all vital for differentiation. Patent protections extending into the next decade would grant exclusivity, allowing premium pricing models.
Regulatory and Reimbursement Outlook
Favorably progressing through clinical trials and regulatory channels could enable early market entry, especially if LO-ZUMANDIMINE demonstrates clear benefits over existing standards. Reimbursement policies will significantly influence market penetration, especially in key territories such as the US, EU, and Japan.
Market Size and Adoption Potential
Global Market Valuation
The global neurodegenerative disease therapeutics market is projected to reach over USD 12 billion by 2028, growing at approximately 8% CAGR ([3]). Taking into account potential indications and age demographics, LO-ZUMANDIMINE could capture a significant segment upon approval.
Market Penetration Scenarios
- Optimistic Scenario: Rapid regulatory approval, high efficacy, early adoption by major healthcare providers, capturing 10-15% of the addressed market within 5 years.
- Conservative Scenario: Lengthier approval timelines, cautious prescriber acceptance, capturing 3-5% of the market over a decade.
These projections hinge on clinical success, pricing strategy, payer negotiations, and competitive dynamics.
Pricing Strategy and Price Projections
Factors Influencing Pricing
LO-ZUMANDIMINE’s pricing will depend on several factors:
- Development costs: High R&D investment and clinical trial expenses.
- Market exclusivity: Patent protections and lack of generic competition initially enable premium pricing.
- Therapeutic value: Demonstrable efficacy and improved safety profiles justify higher prices.
- Reimbursement landscape: Payer willingness to reimburse at premium levels influences achievable prices.
- Manufacturing costs: Economies of scale and supply chain efficiencies will impact margins.
Current Benchmarks
In the neuropharmacology space, drug prices vary widely:
- Brand-name drugs: Can range from USD 2,000 to USD 5,000 per month ([4]).
- Biologics and specialty drugs: Often exceed USD 10,000 per month.
Given LO-ZUMANDIMINE’s potential novel status and therapeutic class, initial annual treatment costs are projected in the USD 30,000–50,000 range, positioning it as a premium yet accessible option.
Price Trajectory Projections
- Year 1 Post-Launch: USD 45,000–USD 50,000 annually, reflecting high development costs and exclusivity.
- Year 3–5: Slight downward pressure to USD 40,000–USD 45,000 due to payer negotiations and volume growth.
- Long Term (10+ years): Potential reduction to USD 30,000–USD 35,000 with the advent of generics or biosimilars, if applicable.
Pricing strategies should incorporate tiered models, value-based pricing, and access negotiations to maximize market penetration.
Regulatory and Market Challenges
- Regulatory hurdles: Pending robust clinical data, delays in approvals could hinder early revenue streams.
- Market acceptance: Physicians’ familiarity with existing therapies and safety profiles play pivotal roles.
- Pricing pressures: Payers demanding value-based assessments may limit peak pricing potential.
- Competition: Emergence of novel pipeline candidates could impact market share.
Strategic Recommendations
- Invest in robust post-market surveillance to substantiate long-term value.
- Develop flexible pricing and access strategies aligned with payers’ expectations.
- Engage early with regulatory agencies to facilitate smooth approval processes.
- Strengthen pipeline by diversifying indications and exploring combination therapies.
Key Takeaways
- Market Opportunity: LO-ZUMANDIMINE targets large, underserved populations with high growth potential, especially in neurodegenerative disorders.
- Competitive Positioning: Its innovative mechanism and early clinical success could position it favorably, provided regulatory milestones are met.
- Pricing Outlook: Premium pricing expectations are justified given the therapeutic benefits, with strategic adjustments to sustain market share over time.
- Risk Factors: Clinical, regulatory, and reimbursement challenges remain; proactive stakeholder engagement is essential.
- Long-term Potential: With successful commercialization, LO-ZUMANDIMINE could command a significant share of the neurotherapeutic market, especially if early adoption metrics align with projections.
FAQs
1. What factors will most influence LO-ZUMANDIMINE’s market success?
Clinical efficacy, regulatory approval timing, reimbursement negotiations, and differentiated therapeutic advantages will be critical. Market acceptance depends on demonstrating clear benefits over existing therapies.
2. How does the current competitive landscape impact LO-ZUMANDIMINE’s pricing strategy?
Intense competition and reimbursement pressures may temper initial pricing, necessitating value-based pricing models that balance profitability with payer demands.
3. What are potential barriers to LO-ZUMANDIMINE’s market entry?
Regulatory delays, safety concerns, physician adoption hesitance, and emerging pipeline drugs could impede rapid market penetration.
4. How might patent protection influence LO-ZUMANDIMINE’s pricing over time?
Strong patent protections allow for premium pricing initially; however, impending patent expirations could lead to generic competition and price reductions.
5. What is the outlook for LO-ZUMANDIMINE beyond 10 years?
If approved and adopted widely, long-term success depends on lifecycle management strategies, including additional indications, biosimilar development, and market expansion into new regions.
Sources
[1] Industry clinical trial reports and press releases.
[2] Market research reports on neurodegenerative disorder therapeutics.
[3] Global Market Insights. Neurodegenerative disease drugs market forecast.
[4] Pricing analysis of neurotherapeutics in the US and EU markets.
More… ↓
