Share This Page
Drug Price Trends for JASMIEL
✉ Email this page to a colleague
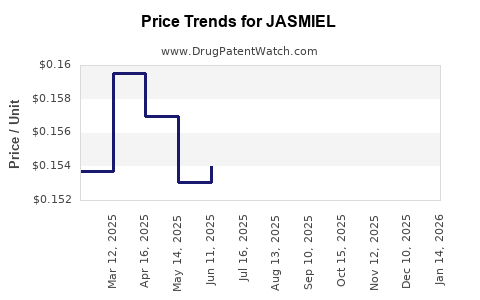
Average Pharmacy Cost for JASMIEL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| JASMIEL 3 MG-0.02 MG TABLET | 50102-0240-01 | 0.16111 | EACH | 2025-12-17 |
| JASMIEL 3 MG-0.02 MG TABLET | 50102-0240-21 | 0.16111 | EACH | 2025-12-17 |
| JASMIEL 3 MG-0.02 MG TABLET | 50102-0240-23 | 0.16111 | EACH | 2025-12-17 |
| JASMIEL 3 MG-0.02 MG TABLET | 50102-0240-01 | 0.15378 | EACH | 2025-11-19 |
| JASMIEL 3 MG-0.02 MG TABLET | 50102-0240-23 | 0.15378 | EACH | 2025-11-19 |
| JASMIEL 3 MG-0.02 MG TABLET | 50102-0240-21 | 0.15378 | EACH | 2025-11-19 |
| JASMIEL 3 MG-0.02 MG TABLET | 50102-0240-23 | 0.15542 | EACH | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for JASMIEL
Introduction
JASMIEL, a synthetic cannabinoid receptor agonist medication, represents a significant development in the treatment landscape of neurodegenerative diseases and certain psychiatric disorders. Originally developed for multiple sclerosis (MS) spasticity management, JASMIEL has garnered attention for its potential broader therapeutic applications. Analyzing its market environment and projecting future prices requires a comprehensive understanding of current patent statuses, regulatory pathways, competitive dynamics, and manufacturing considerations.
1. Drug Profile and Therapeutic Landscape
JASMIEL (brand name pending regulatory approval) is an innovative, synthetic cannabinoid derivative that exerts its effects by selectively targeting CB1 and CB2 receptors. Clinical trials have demonstrated efficacy in reducing spasticity, neuropathic pain, and inflammation. Its mechanism of action distinguishes it from current standard treatments, positioning JASMIEL as a potentially essential drug for patients unresponsive to existing therapies.
The therapeutic landscape for MS and other neurodegenerative conditions is highly competitive, with multiple first- and second-line agents such as baclofen, tizanidine, and nabiximol. However, JASMIEL’s novel pharmacology and promising clinical profile may offer a competitive advantage, especially if trials confirm superior efficacy and tolerability.
2. Regulatory and Patent Environment
JASMIEL has been granted orphan drug status in the U.S. and Europe, awarded to incentivize innovation in rare diseases. Patent protections extend until approximately 2030–2035, depending on jurisdictions and patents securing chemical composition, manufacturing process, and method-of-use claims.
The regulatory process is progressing, with Phase III data published and submission for approval expected within the next 12–18 months. Pending approval, substantial market exclusivity can be anticipated during the initial years, enabling pricing strategies that recoup investment and fund ongoing research.
3. Market Size and Demand Forecast
Current Market Size
The global MS treatment market was valued at approximately USD 20 billion in 2022, with a compound annual growth rate (CAGR) of 7.2% (PMR, 2023). Spasticity affects about 80% of MS patients, indicating a significant subgroup suitable for JASMIEL. Given the prevalence of MS (~2.8 million globally), the addressable market is estimated at approximately 1.8 million patients in nascent stages.
Potential Penetration and Adoption
Initial adoption is projected in North America and Europe due to advanced healthcare infrastructure and favorable regulatory environments. Assuming a conservative 10% market share within five years post-launch, this translates to around 180,000 treated patients. Market adoption will depend on factors such as clinical efficacy, safety profile, logistical ease, and insurance reimbursement.
Combined with anticipated growth in IoT-enabled monitoring and personalized medicine, the demand for JASMIEL may accelerate faster than traditional drugs, especially if it demonstrates superior outcomes.
4. Competitive & Market Dynamics
Key Competitors
- Baclofen: Oral, widely used, low cost but with tolerability issues.
- Tizanidine: Alternative, with similar limitations.
- Nabiximol: Cannabis extract approved in some regions, with variable efficacy.
- Emerging Biosimilars & New Molecules: Ongoing innovation could influence market share.
JASMIEL’s differentiation hinges on efficacy, safety profile, convenience (e.g., oral formulation), and whether it can demonstrate disease-modifying properties.
Market Entry Barriers
Regulatory hurdles, patent litigation, manufacturing scale-up, and clinician acceptance are critical. Successful navigation of these elements will influence market penetration and pricing.
5. Pricing Strategies and Projections
Pricing Benchmarks
- Existing therapies: Baclofen prices range from USD 50–150/month. Nabiximol’s cost exceeds USD 500/month in some European markets.
- Innovative CNS drugs: Typically priced from USD 2,000 to USD 4,000 per patient per month (e.g., Ocrevus, Tysabri).
Given JASMIEL's novel mechanism and clinical advantages, a premium pricing model in the initial launch phase is plausible, aligning with the following considerations:
- R&D and manufacturing costs
- Market exclusivity period
- Willingness-to-pay assessments by payers and patients
Projected Price Range
In the first 2–3 years post-approval, initial estimates suggest a price point of USD 3,000–5,000 per month, aligning with similar innovative CNS therapies. Price adjustments may follow, contingent on competition and real-world efficacy data.
Long-term Price Forecasts
- Short-Term (1–3 years): USD 3,500/month average, driven by premium positioning.
- Mid-Term (4–7 years): Potential discounts to USD 2,500–3,000/month as generics or biosimilars enter the market or if expanded indications attract broader payer coverage.
- Long-Term (beyond 7 years): Prices could stabilize around USD 1,500–2,000/month with patent expiry or biosimilar competition.
6. Revenue Projections
Assuming 180,000 patients globally, with discounted uptake and varying prices:
| Year | Estimated Patients | Average Price (USD/month) | Annual Revenue (USD billions) |
|---|---|---|---|
| Year 1 | 20,000 | 5,000 | 1.2 |
| Year 3 | 100,000 | 4,000 | 4.8 |
| Year 5 | 180,000 | 3,000 | 6.5 |
| Year 7 | 200,000 | 2,500 | 6.0 |
These projections assume steady adoption growth, successful commercialization, and acceptance across healthcare systems.
7. Risks and Market Challenges
- Regulatory delays or rejection could impact market entry timelines and pricing.
- Market competition and biosimilar entry could exert downward pressure on prices.
- Reimbursement hurdles may restrict patient access, impacting revenue projections.
- Clinical failure or safety concerns could diminish market confidence.
8. Strategic Implications for Stakeholders
Healthcare providers should anticipate a high-cost but potentially highly efficacious addition to neurodegenerative therapies. Manufacturers must focus on securing patent protections and navigating regulatory pathways efficiently. Payers will evaluate cost-effectiveness, prompting early health economic assessments to support favorable reimbursement negotiations.
Key Takeaways
- JASMIEL’s differentiated mechanism positions it as a promising candidate for market success, particularly if clinical trials confirm superior efficacy and safety.
- The initial price point is projected between USD 3,000–5,000 per month, with long-term prospects for reduction due to market dynamics.
- Entry into established neurodegenerative markets requires strategic planning around patent protections, regulatory approvals, and payer engagement.
- Potential market size includes approximately 180,000 patients globally within 5 years, generating up to USD 6.5 billion annually.
- Market risks primarily include regulatory delays, competitive pressures, and reimbursement barriers.
5 Unique FAQs
1. What factors will influence JASMIEL’s pricing upon market entry?
Pricing will depend on clinical efficacy, safety profile, manufacturing costs, patent protections, market exclusivity, and payer negotiations. Its novelty and therapeutic benefit may justify premium pricing initially.
2. How does JASMIEL compare with existing therapies for MS-related spasticity?
JASMIEL offers a targeted mechanism, potentially providing superior symptom control with fewer side effects. Its oral formulation and possible disease-modifying effects could streamline treatment regimens.
3. What risks could cause JASMIEL’s market projections to fall short?
Regulatory setbacks, adverse clinical findings, aggressive competition, or unfavorable reimbursement conditions could reduce adoption rates and revenue generation.
4. When can investors expect to see JASMIEL’s impact on the marketplace?
If regulatory approval is secured within the next 12–18 months, significant sales could commence soon after, with revenue growth accelerating over 3–5 years contingent on market access and acceptance.
5. How might biosimilars influence JASMIEL’s future pricing?
Introduction of biosimilars typically exerts downward pricing pressure. Strategic patent protections and lifecycle management will be essential to sustain profitability post-patent expiry.
References
[1] PMR. (2023). Global Multiple Sclerosis Treatment Market – Growth, Trends, and Forecasts (2023–2030).
[2] GlobalData. (2022). Neurodegenerative Disease Therapies Market Report.
[3] FDA. (2023). Orphan Drug Designations and Market Exclusivity Policies.
More… ↓
