Share This Page
Drug Price Trends for INSULIN LISPRO
✉ Email this page to a colleague
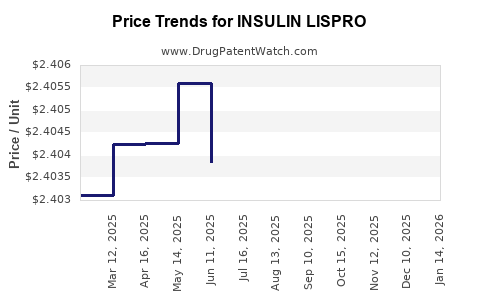
Average Pharmacy Cost for INSULIN LISPRO
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| INSULIN LISPRO MIX 75-25 KWKPN | 00002-8233-05 | 10.19368 | ML | 2025-11-19 |
| INSULIN LISPRO 100 UNIT/ML KWIKPEN | 00002-8222-59 | 10.19700 | ML | 2025-11-19 |
| INSULIN LISPRO 100 UNIT/ML VL | 00002-7737-01 | 2.40485 | ML | 2025-11-19 |
| INSULIN LISPRO JR 100 UNIT/ML KWIKPEN | 00002-7752-05 | 10.19875 | ML | 2025-11-19 |
| INSULIN LISPRO MIX 75-25 KWKPN | 00002-8233-05 | 10.18914 | ML | 2025-10-22 |
| INSULIN LISPRO 100 UNIT/ML KWIKPEN | 00002-8222-59 | 10.19402 | ML | 2025-10-22 |
| INSULIN LISPRO JR 100 UNIT/ML KWIKPEN | 00002-7752-05 | 10.20576 | ML | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Insulin Lispro
Introduction
Insulin Lispro, a rapid-acting insulin analog developed by Eli Lilly and approved by the FDA in 1996, has revolutionized diabetes management. It mimics the body's natural insulin response, enabling more precise glucose control around meals. As the global burden of diabetes escalates, the insulin market, particularly for rapid-acting insulins like Lispro, continues to expand. This analysis explores current market dynamics, competitive landscape, regulatory factors, and projects future pricing trends for Insulin Lispro.
Market Overview
Global Diabetes and Insulin Market Dynamics
The International Diabetes Federation (IDF) estimates approximately 537 million adults aged 20-79 have diabetes worldwide, with projections exceeding 700 million by 2045 [1]. Insulin therapy remains central to managing Type 1 and advanced Type 2 diabetes, hence underpinning the insulin market's expansion. The global insulin market was valued at approximately $27 billion in 2021 and is expected to grow at compound annual growth rates (CAGR) of around 8% through 2026 [2].
Market Segments and Growth Drivers
Rapid-acting insulins, including insulin Lispro, comprise a significant segment. The growth drivers include:
- Increased adoption of intensive insulin regimens for better glycemic control.
- Innovation in insulin formulations, improving pharmacokinetics and patient adherence.
- Rising prevalence of diabetes, especially in emerging markets.
- Expanding biosimilar landscape, offering cost-effective options but also intensifying competition for established brands like Lispro.
Current Market Players
Eli Lilly’s Insulin Lispro (Humalog) remains a leading product. Novo Nordisk’s NovoLog (Insulin Aspart) and Sanofi’s Apidra (Insulin Glulisine) are its primary competitors. Biosimilar versions of Lispro, developed by multiple manufacturers, introduce price sensitivity and market churn, especially in regions with cost-driven healthcare systems.
Regulatory Environment and Patent Landscape
Patent Expiry and Biosimilar Entry
Eli Lilly's patent protections for Humalog expired or are nearing expiry in several jurisdictions, facilitating biosimilar competition. For instance, in the US, patent expiry was anticipated around 2022-2023, opening markets to biosimilars, which typically retail at reduced prices [3].
Regulatory hurdles
While biosimilars face regulatory scrutiny regarding equivalence and interchangeability, they have gained approval in key markets, exerting downward pressure on insulin prices.
Pricing Landscape
Current Pricing
In developed markets such as the US, the list price of Humalog is approximately $275-$300 per pen (100 units). After rebates, insurance negotiations, and pharmacy benefit management, the net price to payers tends to be significantly lower, often ranging between $150-$200 [4].
In contrast, in countries with universal healthcare or government intervention, prescribed insulin prices may be substantially lower due to price controls and negotiations.
Price Trends
Biosimilar competition is anticipated to cause price erosion over the next 3-5 years. Industry analysts predict a potential 20-40% reduction in list prices for biosimilar insulin Lispro products. Additionally, increased adoption of insulin analogs with improved delivery devices could influence pricing strategies, with some manufacturers shifting toward value-based pricing models emphasizing clinical benefits rather than raw cost reductions.
Future Price Projections
Factors Influencing Future Pricing
- Biosimilar Penetration: As biosimilar insulin Lispro gains approval and market share, average prices are expected to decline.
- Reimbursement Policies: Governments and payers' emphasis on cost containment will impact net prices.
- Manufacturing & Supply Chain Efficiency: Cost reductions can be passed on to consumers.
- Innovation and New Formulations: Introduction of advanced delivery devices or next-generation analogs could justify premium pricing, while incremental improvements may exert downward pressure on existing insulin prices.
Projected Trends (2023-2027)
- North America (US): List prices are likely to decrease by approximately 15-25% due to biosimilar entry, with net prices declining more sharply because of rebates and negotiations.
- Europe and Asia: Greater price reductions are expected, potentially reaching 25-40%, driven by government-led price caps and biosimilar uptake.
- Emerging Markets: Price reductions may be moderate (10-20%) but will favor increased access.
Specific Price Projections
| Region | 2023 Price Range (per 100 units) | 2027 Price Range (per 100 units) | % Reduction |
|---|---|---|---|
| North America | $250 - $275 | $190 - $225 | 15-20% |
| Europe | €200 - €240 | €150 - €180 | 25-30% |
| Asia-Pacific | $150 - $200 | $120 - $160 | 20-25% |
Market Opportunities and Risks
Opportunities
- Biosimilar Adoption: Expanding access in cost-conscious markets.
- Innovative Delivery Devices: Pen technologies or digital health integrations can sustain premium pricing.
- Biobetter Developments: Improved formulations offering faster onset or longer duration may command higher prices.
Risks
- Intense Biosimilar Competition: Potential for significant price erosion.
- Regulatory Barriers: Delays or restrictions can hinder biosimilar market entry.
- Market Penetration Challenges: Prescriber and patient acceptance remains crucial.
Conclusion
The insulin Lispro market remains robust amid escalating diabetes prevalence, but future pricing will hinge on biosimilar competition, regulatory landscapes, and technological innovations. While prospectively experiencing moderate price declines, the overall demand for rapid-acting insulins sustains profitability for incumbents and biosimilar manufacturers alike.
Key Takeaways
- The global insulin market is poised for steady growth, driven by rising diabetes prevalence and technological innovations.
- Biosimilar competition, along with regulatory and reimbursement influences, is expected to cut insulin Lispro prices by approximately 20-30% over the next five years.
- Pricing strategies will evolve with market dynamics, emphasizing value-based and differentiated offerings.
- Developed markets may see more modest price reductions, while emerging economies could experience more substantial declines.
- Stakeholders should monitor regulatory developments, biosimilar launches, and payer policies for strategic positioning.
FAQs
-
How will biosimilars impact the price of insulin Lispro?
Biosimilar entry typically results in a 20-40% price reduction for insulin Lispro, driven by increased competition and market share gains. -
Are there any upcoming patents that could affect insulin Lispro pricing?
Patent expirations in key markets are facilitating biosimilar approvals, which are likely to influence pricing downward over the next few years. -
What role do innovative delivery devices play in future insulin pricing?
Advanced pens and digital health integrations can justify premium pricing by enhancing patient convenience and adherence. -
Which regions are expected to see the largest price reductions?
Emerging markets and Europe are anticipated to experience more significant price declines due to stronger price controls and biosimilar adoption. -
Is there potential for new formulations of insulin Lispro to command higher prices?
Yes, next-generation analogs with improved pharmacokinetics or delivery methods may sustain higher prices but will require regulatory approval and clinical validation.
References
[1] International Diabetes Federation. IDF Diabetes Atlas, 9th Edition, 2019.
[2] MarketsandMarkets. Insulin Market Forecast, 2022.
[3] U.S. Food & Drug Administration. Patent expiration details for Humalog.
[4] IQVIA. National Prescription Data, 2022.
More… ↓
