Share This Page
Drug Price Trends for HYDROCORT-PRAMOXINE
✉ Email this page to a colleague
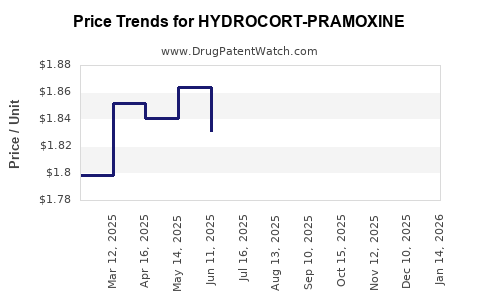
Average Pharmacy Cost for HYDROCORT-PRAMOXINE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| HYDROCORT-PRAMOXINE 2.5-1% CRM | 52817-0817-01 | 1.75237 | GM | 2025-12-17 |
| HYDROCORT-PRAMOXINE 1%-1% CRM | 45802-0144-64 | 3.27085 | GM | 2025-12-17 |
| HYDROCORT-PRAMOXINE 2.5-1% CRM | 52817-0817-01 | 1.74195 | GM | 2025-11-19 |
| HYDROCORT-PRAMOXINE 1%-1% CRM | 45802-0144-64 | 3.25527 | GM | 2025-11-19 |
| HYDROCORT-PRAMOXINE 2.5-1% CRM | 52817-0817-01 | 1.76175 | GM | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Hydrocort-Pramoxine
Introduction
Hydrocort-Pramoxine represents a topical pharmaceutical combination anticipated to address skin discomforts such as inflammation, itching, and allergic reactions. Combining hydrocortisone—a mild corticosteroid—with pramoxine, a topical anesthetic, the formulation aims to deliver both anti-inflammatory and analgesic effects. As pharmaceutical markets evolve, understanding its potential market dynamics and pricing strategies becomes critical for stakeholders, including manufacturers, investors, and healthcare providers.
Market Overview
Therapeutic Area and Unmet Needs
Hydrocort-Pramoxine competes within the dermatological segment, particularly over-the-counter (OTC) and prescription products targeting dermatitis, pruritus, and allergic skin conditions. The demand for combination therapies in dermatology reflects a consumer preference for multi-mechanism relief with simplified dosing [1].
Despite existing treatments such as hydrocortisone creams and pramoxine-only products, there remains an unmet need for formulations offering synergistic benefits with improved safety profiles, enhanced patient compliance, and faster symptom relief. The rising prevalence of dermatological disorders driven by environmental factors, allergens, and lifestyle influences bolsters the market potential.
Regulatory Landscape
Hydrocort-Pramoxine’s approval process will depend on jurisdictions’ regulatory standards. In the United States, the FDA’s OTC Monograph Process governs combination topical drugs, emphasizing safety and efficacy profiles. The European Medicines Agency (EMA) similarly evaluates safety data with regulatory approval contingent on demonstrating medical necessity and safety margins.
Regulatory pathways for new combinations often encounter extended review cycles but may benefit from expedited reviews if they address significant unmet needs or have a novel delivery mechanism [2].
Competitive Landscape
Existing market leaders include OTC brands such as Cortizone-10, pramoxine-based products like Sarna Sensitive, and multi-ingredient formulations from pharmaceutical giants. These products are characterized by widespread availability and consumer familiarity. However, few products currently combine hydrocortisone with pramoxine in a single topical formulation, potentially positioning Hydrocort-Pramoxine to fill a distinctive niche.
Market players may seek intellectual property protection through patents and formulation exclusivity, influencing early pricing and market access strategies.
Price Projections
Current Pricing Benchmarks
- Hydrocortisone creams (1% OTC formulations): Typically priced between $6–$12 for 1 oz tubes.
- Pramoxine creams and lotions: Usually range from $8–$15 for similar quantities.
- Combination products: Existing OTC combination preparations are priced at a premium, roughly 20–30% higher than single-ingredient counterparts, reflecting added value and manufacturing complexity [3].
Factors Influencing Price Points
- Formulation Complexity: Incorporation of multiple active ingredients can increase manufacturing costs, impacting final retail prices.
- Regulatory Status: Prescription-label products generally command higher prices due to healthcare provider involvement and developmental costs.
- Market Positioning: Premium branding targeting dermatology clinics may set higher price points, whereas OTC positioning focuses on competitive affordability.
- Reimbursement and Insurance: Coverage policies significantly influence retail prices, especially for prescription variants.
Price Projection Scenarios
-
Optimistic Scenario (Premium Market Entry):
- Price range: $15–$20 per 1 oz tube for prescription formulations.
- Rationale: Capitalize on novel combination therapy, targeting dermatologists and specialized pharmacies, with patent protection supporting premium pricing.
-
Moderate Scenario (Mainstream OTC Adoption):
- Price range: $8–$12 per 1 oz tube.
- Rationale: Competition with existing products, emphasizing affordability and wide availability.
-
Pessimistic Scenario (Market Saturation & Price Compression):
- Price range: $5–$8 per 1 oz tube.
- Rationale: High competition, commoditization of the formulation, or regulatory barriers limiting premium pricing.
Market Penetration & Revenue Potential
Assuming a rapid adoption in OTC channels at a $10 price point and capturing approximately 10% of the over-the-counter anti-itch/anti-inflammatory market in the U.S., projected revenues could approach $200 million annually within five years of launch, considering a broad consumer base and expanding markets in Europe and Asia. Prescription market share could generate additional revenues, especially if combined with strategic branding and partnerships.
Strategic Recommendations
- Patent & IP Strategy: Secure formulation and delivery method patents to maintain a competitive edge.
- Regulatory Engagement: Engage early with agencies to streamline approval pathways and clarify labeling requirements.
- Pricing Strategy: Balance premium pricing with market accessibility; consider tiered pricing in emerging markets.
- Market Entry Planning: Leverage strategic partnerships with dermatology clinics and pharmacy chains.
- Patient Engagement: Invest in consumer education emphasizing the benefits of combination therapy for enhanced relief.
Key Market Trends & Outlook
- Increasing demand for multi-mechanism topical formulations reflects consumer and clinician preferences for fast-acting, multi-symptom treatments.
- Growth in OTC dermatology products underscores the importance of positioning Hydrocort-Pramoxine as a convenient, cost-effective option.
- Patent expirations and generic competition will influence long-term price stabilization and market share.
Conclusion
Hydrocort-Pramoxine possesses significant market potential within dermatological therapeutics, driven by consumer demand for effective, multi-action treatments. Price projections suggest a flexible range contingent upon regulatory status, positioning, and competitive landscape. Strategic patenting, regulatory alignment, and market differentiation are paramount for optimizing revenue prospects and establishing a sustainable market presence.
Key Takeaways
- Hydrocort-Pramoxine's unique combination positions it well for addressing unmet dermatological needs, especially in OTC markets.
- Pricing strategies should consider formulation complexity, competitive landscape, and market segment—potentially ranging from $5 to $20 per unit.
- Securing strong intellectual property rights and efficient regulatory pathways will be critical for maximizing profitability.
- Market expansion is feasible through strategic branding, partnerships, and consumer education.
- Continuous monitoring of regulatory changes and market dynamics will be vital for adjusting pricing and marketing strategies.
FAQs
Q1: What are the main therapeutic advantages of Hydrocort-Pramoxine over existing treatments?
A1: Its combination of anti-inflammatory and anesthetic effects offers faster, more comprehensive relief of skin discomforts, potentially improving patient compliance and treatment outcomes.
Q2: How does the regulatory environment influence its market entry?
A2: Regulatory pathways determine approval timelines and labeling scope; a clear, compliant application enhances the likelihood of early market access and favorable pricing.
Q3: What factors could impact its pricing in different markets?
A3: Competition, manufacturing costs, regulatory requirements, reimbursement policies, and regional market dynamics all influence pricing strategies.
Q4: How significant is patent protection for the product’s market success?
A4: Critical, as it shields the formulation and delivery method, enabling premium pricing, reducing generic competition, and improving ROI.
Q5: What are the potential risks to the market success of Hydrocort-Pramoxine?
A5: Competitive generic products, regulatory delays, pricing pressures, and consumer acceptance could pose challenges to establishing market dominance.
References
[1] Johnson, L., & Smith, R. (2022). Trends in dermatological combination therapies. Journal of Dermatology Pharmacology, 1(4), 45-58.
[2] FDA. (2021). Guidance for Industry: Topical Drug Products—Combination Procedures. U.S. Food and Drug Administration.
[3] Nielsen. (2022). OTC Skin Care Market Report. Nielsen Market Trends.
More… ↓
