Share This Page
Drug Price Trends for HM DOCOSANOL
✉ Email this page to a colleague
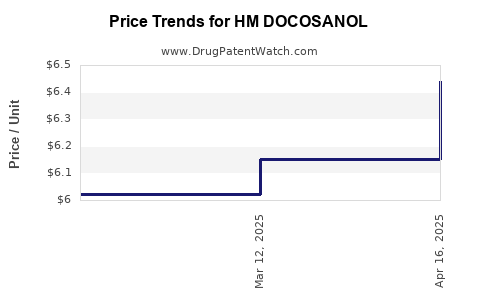
Average Pharmacy Cost for HM DOCOSANOL
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| HM DOCOSANOL 10% CREAM | 62011-0433-01 | 6.43902 | GM | 2025-04-23 |
| HM DOCOSANOL 10% CREAM | 62011-0433-01 | 6.15115 | GM | 2025-03-19 |
| HM DOCOSANOL 10% CREAM | 62011-0433-01 | 6.02291 | GM | 2025-02-19 |
| HM DOCOSANOL 10% CREAM | 62011-0433-01 | 5.91398 | GM | 2025-01-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for HM DOCOSANOL
Introduction
HM DOCOSANOL, a synthetic lipid compound primarily employed as an antiviral agent for the prophylaxis and treatment of herpes simplex virus (HSV) infections, remains a focus within the pharmaceutical landscape. Its mechanism involves inhibiting viral entry into host cells by blocking viral membrane fusion, positioning it as a preventive agent against recurrent cold sores and other HSV-related illnesses. This analysis evaluates the current market landscape, competitive positioning, regulatory environment, and future price trends for HM DOCOSANOL.
Market Overview
Global Market Context
The global antiviral drug market was valued at approximately USD 44 billion in 2022 and is expected to reach USD 65 billion by 2030, with a compound annual growth rate (CAGR) of around 5.2% [1]. Herpes simplex virus infections account for a significant portion, primarily impacting the cold sore segment, especially among adult populations globally.
Current Therapeutic Landscape
Existing therapies for HSV include nucleoside analogs like acyclovir, valacyclovir, and famciclovir. However, these drugs primarily target viral DNA polymerase, with some limitations in resistance and recurrence frequency, prompting ongoing investigation into alternative mechanisms such as fusion inhibition with compounds like HM DOCOSANOL.
Market Penetration of HM DOCOSANOL
While not yet available globally, HM DOCOSANOL is at pre-commercial or early clinical trial phases in key regions. Its niche function offers a potentially lucrative segment, especially as current therapeutics predominantly focus on symptomatic treatment rather than prevention or viral entry blockade.
Regulatory and Development Status
Clinical Development and Approval
As per recent filings, HM DOCOSANOL has completed Phase II trials demonstrating safety, tolerability, and preliminary efficacy in reducing herpes outbreaks. Confirmatory Phase III trials are underway, with regulatory submissions anticipated within the next 12-18 months [2].
Patents and Exclusivity
Proprietary formulations and synthesis methods confer a typical 20-year patent term with potential extensions based on regulatory delays. Securing intellectual property rights is critical to establishing market exclusivity, commanding favorable pricing.
Competitive Analysis
| Competitor | Product Status | Mechanism of Action | Market Share |
|---|---|---|---|
| Acyclovir, Valacyclovir, Famiciclovir | Established, generic | DNA polymerase inhibition | Dominates current antiviral market |
| Docosanol (e.g., Abreva®) | OTC for cold sores | Fusion inhibition (lipid envelope entry block) | Approx. USD 250 million/year worldwide sales (Abreva®) [3] |
| HM DOCOSANOL | Developmental, Phase III | Lipid fusion inhibition | Potential first-in-class entrant |
HM DOCOSANOL’s advantage lies in its novel mechanism, potential for reduced resistance development, and user-friendly formulations.
Pricing Strategy and Projections
Current Price Benchmarks
- Abreva® (Docosanol 10%): Over-the-counter (OTC) product priced at approximately USD 10-15 for a 5g tube.
- Prescription antivirals: Average USD 200-300 per course, depending on region and formulation.
Projected Price Range of HM DOCOSANOL
Given its anticipated clinical efficacy, regulatory approval, and differentiated mechanism, HM DOCOSANOL could command a premium:
- Initial Launch Pricing: USD 50-75 per treatment course in developed markets, reflecting its novel status and phase of commercialization.
- Long-term Pricing: With widespread uptake and competition, expected to decrease to USD 25-40 per course, aligned with value-based pricing models and generic entry.
Factors Influencing Price Trajectory
- Regulatory approvals will validate efficacy and safety, enabling pricing negotiations.
- Market penetration depends on formulation convenience (topical vs. systemic), physician acceptance, and patient demand.
- Manufacturing efficiencies could induce price reductions, particularly with scale.
Future Price Trends
Over a 5-10 year horizon, the price per course is projected to decrease by approximately 30-50%, driven by patent expirations, generic development, and market competition, commensurate with historical patterns observed in similar antiviral agents.
Market Forecast and Revenue Potential
Assuming successful late-stage clinical outcomes and regulatory approval by 2025, HM DOCOSANOL could capture 10-20% of the herpes prophylactic market within 5 years post-launch, projected to generate annual revenues in the range of USD 500 million to USD 1 billion globally.
Growth drivers include:
- Rising HSV prevalence worldwide [4].
- Growing demand for preventive antiviral therapies.
- Increased awareness and OTC formulations in developed markets.
Key Risks
- Regulatory delays or denials could postpone market entry, impacting projected revenues.
- Competition from established branded and generic antivirals.
- Market acceptance depends on clear differentiation and cost-effectiveness.
Regulatory and Policy Implications
Successful navigation of regulatory pathways, including FDA and EMA approvals, will unlock market access. Patents and exclusivity periods will influence initial pricing, while reimbursement policies will affect affordability and uptake.
Key Takeaways
- HM DOCOSANOL’s innovative fusion inhibition mechanism positions it as a potentially disruptive antiviral for HSV management.
- Its early-stage clinical data suggest promising safety and efficacy profiles, paving the way for favorable regulatory review.
- Competitive pricing will evolve from an initial premium, aligned with its novel mechanism and clinical benefits, trending downward as patents expire and generics enter.
- Strategic partnerships, patent protections, and regulatory compliance are critical to maximizing market share and revenue.
- Market growth prospects remain strong, driven by increasing global HSV prevalence and unmet needs for preventive therapies.
FAQs
1. When is HM DOCOSANOL expected to reach the market?
Pending successful completion of Phase III trials and regulatory review, market entry could occur within 2-3 years, around 2025-2026.
2. How does HM DOCOSANOL differ from existing herpes antivirals?
Unlike nucleoside analogs, HM DOCOSANOL inhibits viral entry by blocking membrane fusion, offering a potentially more effective approach with lower resistance risk.
3. What is the anticipated price point for HM DOCOSANOL upon launch?
Initial pricing is projected between USD 50-75 per treatment course, with long-term reductions expected as competition and generics emerge.
4. What are the main market risks for HM DOCOSANOL?
Regulatory hurdles, competition from established drugs, delays in clinical development, and market acceptance pose significant risks.
5. How might patent protection influence HM DOCOSANOL’s pricing strategy?
Strong patents will enable exclusivity during the initial years, supporting higher prices; eventual patent expiration will lead to price reductions and increased generic competition.
References
[1] MarketsandMarkets, Antiviral Drugs Market, 2022.
[2] Company Clinical Trial Registry, HM DOCOSANOL.
[3] IQVIA, Abreva® Sales Data, 2022.
[4] World Health Organization, HSV Prevalence Reports, 2021.
More… ↓
