Drug Price Trends for GABAPENTIN
✉ Email this page to a colleague
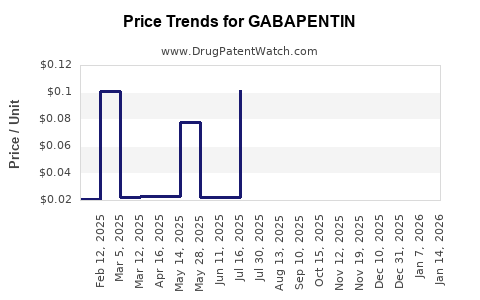
Average Pharmacy Cost for GABAPENTIN
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| GABAPENTIN 800 MG TABLET | 82009-0072-05 | 0.10341 | EACH | 2024-04-17 |
| GABAPENTIN 600 MG TABLET | 82009-0071-05 | 0.08652 | EACH | 2024-04-17 |
| GABAPENTIN 800 MG TABLET | 76282-0707-90 | 0.10341 | EACH | 2024-04-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Best Wholesale Price for GABAPENTIN
| Drug Name | Vendor | NDC | Count | Price ($) | Price/Unit ($) | Unit | Dates | Price Type |
|---|---|---|---|---|---|---|---|---|
| GABAPENTIN 250MG/5ML SOLN,ORAL | Golden State Medical Supply, Inc. | 42192-0608-45 | 40X5ML | 135.22 | 2023-06-15 - 2028-06-14 | FSS | ||
| GABAPENTIN 300MG CAP | Golden State Medical Supply, Inc. | 51407-0048-90 | 90 | 6.69 | 0.07433 | EACH | 2023-11-15 - 2028-06-14 | FSS |
| GABAPENTIN 250MG/5ML SOLN,ORAL | Golden State Medical Supply, Inc. | 50383-0311-47 | 470ML | 80.00 | 0.17021 | ML | 2023-06-15 - 2028-06-14 | FSS |
| >Drug Name | >Vendor | >NDC | >Count | >Price ($) | >Price/Unit ($) | >Unit | >Dates | >Price Type |


