Share This Page
Drug Price Trends for FT ITCH RLF WITH ALOE
✉ Email this page to a colleague
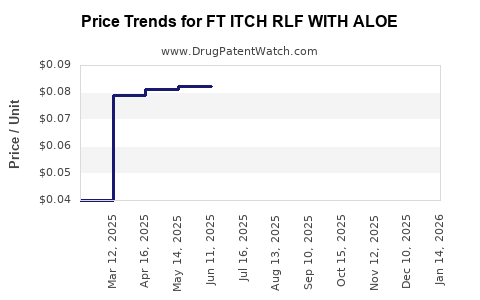
Average Pharmacy Cost for FT ITCH RLF WITH ALOE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| FT ITCH RLF WITH ALOE 1% CREAM | 70677-1215-01 | 0.07428 | GM | 2025-12-17 |
| FT ITCH RLF WITH ALOE 1% CREAM | 70677-1216-01 | 0.07428 | GM | 2025-12-17 |
| FT ITCH RLF WITH ALOE 1% CREAM | 70677-1216-02 | 0.04000 | GM | 2025-12-17 |
| FT ITCH RLF WITH ALOE 1% CREAM | 70677-1215-01 | 0.07331 | GM | 2025-11-19 |
| FT ITCH RLF WITH ALOE 1% CREAM | 70677-1216-02 | 0.04000 | GM | 2025-11-19 |
| FT ITCH RLF WITH ALOE 1% CREAM | 70677-1216-01 | 0.07331 | GM | 2025-11-19 |
| FT ITCH RLF WITH ALOE 1% CREAM | 70677-1216-02 | 0.04000 | GM | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for FT ITCH RLF with Aloe
Introduction
The pharmaceutical and consumer health markets have witnessed significant growth in anti-itch and skincare products containing natural ingredients like aloe vera. FT ITCH RLF with Aloe, a topical formulation targeting itch relief and skin soothing, occupies a niche within both dermatological and consumer wellness sectors. This report provides an in-depth market analysis, competitive landscape overview, regulatory considerations, and comprehensive price projections to inform strategic decision-making.
Product Overview
FT ITCH RLF with Aloe combines anti-itch active ingredients with aloe vera extract, known for its skin-healing and soothing properties. The formulation aims to target consumers seeking fast, natural relief from itching caused by insect bites, dermatitis, eczema, and other minor skin irritations. Market positioning emphasizes its natural composition, efficacy, and gentle profile catering to both adult and pediatric users.
Market Landscape
Global Market Size and Trends
The global anti-itch or antipruritic market was valued at approximately USD 1.4 billion in 2022, projected to expand at a CAGR of 4.8% through 2030. The rising prevalence of skin conditions, increased consumer preference for natural remedies, and growing awareness of skincare health propel this expansion[^1].
Key Market Segments
- Dermatological products: Prescription and OTC treatments for eczema, dermatitis, insect bites.
- Consumer health products: Aloe-based lotions, creams, and topical reliefs gaining popularity for their natural appeal.
- Geographic considerations: North America and Europe dominate due to high consumer spending and awareness, while Asia-Pacific offers significant growth potential driven by population size and skincare trends[^2].
Competitive Landscape
Major players include Johnson & Johnson (Benadryl Itch Stopping Cream), Aveeno (Soothing Relieve Neem & Aloe), and CVS Health OTC brands. These incumbents emphasize natural formulations, positioning FT ITCH RLF with Aloe as a differentiated product with potential for niche market capture[^3].
Regulatory Environment
The regulatory landscape for anti-itch products varies globally, with stringent oversight in the U.S. via the FDA’s OTC monograph pathway and similar frameworks in Europe and Asia. For products with natural ingredients like aloe, claims must be substantiated, and quality standards adhered to, influencing pricing and market entry strategies[^4].
Market Drivers and Challenges
Drivers
- Increasing incidence of skin allergies and sensitivities.
- Consumer shift towards natural and organic products.
- Expansion of OTC and self-care segments.
- Enhanced awareness of aloe's benefits.
Challenges
- Regulatory hurdles impacting formulation and claims.
- Competition from established brands.
- Variability in aloe quality and sourcing.
- Price sensitivity among target consumers.
Price Analysis
Pricing Strategy
The estimated retail price range for FT ITCH RLF with Aloe reflects its positioning as a premium, natural anti-itch treatment:
- Entry-level OTC products: USD 5 - 7 for 50g tubes.
- Premium formulations: USD 8 - 12 for comparable sizes, owing to natural ingredients and branding.
Historical Prices & Market Position
While similar products exist, formulations with added aloe vera tend to command a premium of approximately 20-30% over generic anti-itch creams, accounting for ingredient costs and consumer perception[^5].
Cost Components
- Raw Materials: Aloe vera extract, anti-itch actives, excipients.
- Manufacturing: Quality control, packaging, labeling.
- Distribution: Retail margins, online sales channels.
- Regulatory & Marketing: Advertising, claims substantiation.
The expected wholesale cost per unit typically ranges from USD 1.50 to 2.50, with retail margins set at approximately 40-50%.
Market Projections and Price Trends
Projected Sales Volumes
Assuming successful market entry targeting North American and European markets, initial sales projections for year one estimate approximately 2 million units, driven by OTC retail channels, online platforms, and pharmacy chains.
With increasing consumer acceptance and expanding distribution, sales volume could grow at a CAGR of 7-10% over five years.
Price Trajectory
- Short-term (Years 1-2): Maintaining premium pricing (~ USD 10) to establish brand recognition.
- Mid-term (Years 3-5): Potential price adjustments downward (~ USD 8-9) with economies of scale and increased competition.
- Long-term (Year 5+): Introduce variant sizes (e.g., travel packs, family packs) and formulations to diversify revenue.
Price elasticity among consumers suggests a moderate sensitivity; maintaining perceived value through efficacy and natural appeal is critical.
Strategic Recommendations
- Focus on branding that emphasizes natural ingredients and efficacy.
- Leverage online and direct-to-consumer channels to maximize margins.
- Monitor regulatory developments to avoid compliance costs.
- Expand product line with complementary skincare items to foster brand loyalty.
Key Takeaways
- The anti-itch and natural skincare markets are poised for sustained growth, with Aloe-infused formulations occupying a lucrative niche.
- Competitive differentiation hinges on efficacy, natural ingredient sourcing, and regulatory compliance.
- Pricing strategies should balance premium positioning with consumer willingness to pay, considering market segments.
- Volume growth is achievable through strategic distribution and marketing, with forecasted sales escalating by 7-10% annually.
- Variants, size options, and line extensions can sustain long-term revenue growth and competitive advantage.
FAQs
1. What is the typical retail price range for aloe-based anti-itch creams?
Retail prices generally range from USD 5 to USD 12 per 50g tube, with premium formulations commanding higher prices due to natural ingredients and branding.
2. How does aloe vera influence the marketability of anti-itch products?
Aloe vera’s proven soothing and skin-healing properties enhance consumer perception, allowing brands to position products as natural, gentle, and effective, thereby supporting higher price points.
3. What are key regulatory considerations for launching FT ITCH RLF with Aloe?
Regulatory compliance involves substantiating claims about safety and efficacy, adhering to GMP standards, and navigating OTC monograph pathways or equivalent regional regulations.
4. Which markets present the highest growth opportunities for this product?
North America and Europe lead in market maturity, but Asia-Pacific offers significant growth potential driven by rising consumer demand for natural skincare solutions.
5. What strategies can optimize profit margins for FT ITCH RLF with Aloe?
Prioritize direct channels, scale manufacturing, utilize premium branding, and expand product variants to increase margins and market penetration.
References
[^1]: Statista, "Global Anti-Itch Market Size," 2022.
[^2]: MarketsandMarkets, "Skincare Market Trends," 2022.
[^3]: IBISWorld, "Over-the-Counter & Consumer Healthcare Industry," 2022.
[^4]: FDA, "OTC Monograph Process," 2022.
[^5]: DirectIndustry, "Pricing Trends in Natural Skincare," 2021.
More… ↓
