Share This Page
Drug Price Trends for FT ENEMA READY TO USE TWIN PAK
✉ Email this page to a colleague
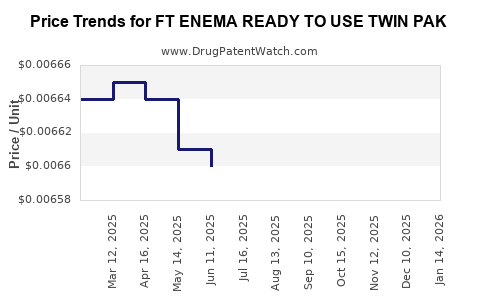
Average Pharmacy Cost for FT ENEMA READY TO USE TWIN PAK
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| FT ENEMA READY TO USE TWIN PAK | 70677-1089-02 | 0.00662 | ML | 2025-12-17 |
| FT ENEMA READY TO USE TWIN PAK | 70677-1089-02 | 0.00662 | ML | 2025-11-19 |
| FT ENEMA READY TO USE TWIN PAK | 70677-1089-02 | 0.00662 | ML | 2025-10-22 |
| FT ENEMA READY TO USE TWIN PAK | 70677-1089-02 | 0.00662 | ML | 2025-09-17 |
| FT ENEMA READY TO USE TWIN PAK | 70677-1089-02 | 0.00661 | ML | 2025-08-20 |
| FT ENEMA READY TO USE TWIN PAK | 70677-1089-02 | 0.00660 | ML | 2025-07-23 |
| FT ENEMA READY TO USE TWIN PAK | 70677-1089-02 | 0.00660 | ML | 2025-06-18 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for FT ENEMA READY TO USE TWIN PAK
Introduction
The pharmaceutical sector continues to expand, driven by increasing awareness of gastrointestinal health and expanding therapeutic applications of enema-based products. FT ENEMA READY TO USE TWIN PAK represents a specific segment within this market—ready-to-use enema solutions designed for convenience, compliance, and improved patient outcomes. This analysis explores current market dynamics, competitive landscape, regulatory considerations, and future price projections to inform stakeholders.
Market Overview
Market Size and Growth Drivers
The global enema market is projected to reach approximately $600 million by 2027, growing at a compound annual growth rate (CAGR) of around 4.2% from 2022 to 2027 [1]. The demand is primarily fueled by an increasing prevalence of constipation, bowel disorders, and gastrointestinal diseases, especially among aging populations and patients with chronic conditions.
Specifically, the segment for ready-to-use formulations like FT ENEMA's Twin PAK—premixed and pre-packaged for convenience—is gaining prominence. Its ease of use enhances patient adherence and minimizes application errors, spiking demand in institutional settings, long-term care facilities, and outpatient clinics.
Market Segmentation
- By Product Type: Liquid enemas, foam, micro-enemas.
- By Application: Constipation relief, bowel cleansing, diagnostic procedures.
- By End-User: Hospitals, clinics, pharmacies, home care.
The twin-pak format — comprising two pre-measured doses — specifically appeals to users seeking convenience and dose precision, especially in self-administered settings.
Competitive Landscape
Key Players
- Abbott Laboratories (e.g., Fleet enema)
- B. Braun Melsungen AG
- Sun Pharmaceutical Industries Ltd.
- Pfizer Inc.
- Fresenius Kabi
FT ENEMA's key differentiator lies in its ready-to-use Twin PAK format, which enhances ease of administration and compliance. The product's unique packaging and pre-measured doses position it favorably against traditional, multi-step enema solutions.
Market Penetration Strategies
- Partnerships with healthcare providers to promote prefixed doses.
- Targeted marketing in geriatrics and pediatric segments.
- Regulatory approvals to expand into new geographic markets.
Regulatory Environment
Regulatory approval processes vary by region but generally require demonstrating safety, efficacy, and quality. The FDA classifies enema solutions as combination products with specific guidelines (21 CFR Part 3), while the EMA maintains stringent standards under the Medical Devices Regulation.
FT ENEMA's compliance with Good Manufacturing Practices (GMP) and solid clinical data underpin market acceptance and facilitate expansion into different jurisdictions. Emerging regulations emphasizing patient safety and convenience will influence pricing and market penetration strategies.
Pricing Analysis and Projections
Current Price Landscape
The price of enema products varies globally, influenced by manufacturing costs, packaging, branding, and distribution channels. Basic generic enema formulations typically retail between $3-$8 per single-use unit, while branded or specialty formulations like FT ENEMA's Twin PAK command premium prices of $12-$20 per package.
In developed markets, ready-to-use twin-packs are priced at approximately $15–$25 for a pack of two doses, reflecting added convenience, quality, and branding premiums.
Factors Influencing Price
- Manufacturing Costs: Advanced materials for single-use, sterile packaging, compliance expenses.
- Regulatory Costs: Certification, clinical trial expenses.
- Market Demand: Growing preference for convenient, pre-measured doses.
- Competitive Positioning: Premium branding often allows for higher margins.
Projected Price Trends (2023–2030)
Over the next decade, several factors are expected to influence pricing trajectories:
- Economies of scale as production ramps up can lead to reduced manufacturing costs, allowing for competitive pricing strategies.
- Increased competition from generic and private-label alternatives could exert downward pressure, particularly in price-sensitive markets.
- Regulatory developments requiring higher safety standards may temporarily inflate costs but could evoke premiums due to perceived quality assurance.
- Innovations in packaging and formulation may justify premium pricing for convenience-focused products.
Based on these dynamics, the price of FT ENEMA READY TO USE TWIN PAK is projected to:
- Remain stable or slightly decline in mature markets due to competitive pressures and increased production efficiency.
- Have a potential premium of 10-15% over traditional formulations, owing to its ready-to-use design, safety, and compliance features.
- Evolve within a range of $12–$20 per twin pack through 2030, with regional variations.
Regional Variations
- North America: Higher willingness to pay for convenience could sustain prices toward the upper end of the range.
- Europe: Regulatory and reimbursement policies may moderate price increases.
- Asia-Pacific: Price sensitivity is higher; local manufacturing and generics could exert downward pressure, possibly reducing prices to $8–$12.
Market Entry and Price Optimization Strategies
- Establish collaborations with healthcare providers to embed FT ENEMA in clinical protocols, supporting premium pricing.
- Leverage pharmacoeconomic studies demonstrating cost-saving benefits via improved adherence and reduced complications.
- Tailor packaging formats and pricing models to different regional market conditions.
Risks and Challenges
- Pricing erosion due to intense competition and market saturation.
- Regulatory hurdles in emerging markets potentially delaying product launch and impacting pricing.
- Patient acceptance concerns regarding pre-measured doses versus traditional dosing methods.
Key Takeaways
- The global enema market is poised for steady growth, with a notable shift towards convenience-enhanced formulations like FT ENEMA's Twin PAK.
- Competitive pricing will depend on manufacturing scalability, regulatory compliance, and strategic positioning, with anticipated price ranges of $12–$20 per twin pack.
- Innovative packaging, clinical efficacy, and targeted marketing are essential for capturing premium segments.
- Regional market dynamics, including healthcare infrastructure and reimbursement policies, significantly influence pricing strategies.
- A proactive approach toward regulatory approval and alliance building will optimize market share and pricing potential.
Conclusion
FT ENEMA READY TO USE TWIN PAK operates within a favorable market landscape driven by aging populations, rising gastrointestinal health awareness, and consumer preference for convenience. While competitive pressures threaten to compress prices, strategic positioning, quality assurance, and regional tailoring can sustain premium pricing. Stakeholders should focus on regulatory alignment, market penetration strategies, and demonstrating value to maximize profitability in this expanding sector.
FAQs
-
What are the main advantages of FT ENEMA's Twin PAK compared to traditional enema solutions?
Its pre-measured, ready-to-use design enhances patient compliance, simplifies administration, and reduces dosing errors, making it ideal for self-administration and clinical settings. -
How does regional regulation influence the pricing of enema products?
Strict regulatory standards and approval costs in regions like the EU and US can elevate initial product prices, whereas less regulatory overhead in emerging markets may lead to more competitive pricing. -
What factors could cause a decline in the price of FT ENEMA's Twin PAK?
Increased market competition, manufacturing cost reductions, and biosimilar or generic entries could exert downward pressure on pricing. -
Will the demand for ready-to-use enema products increase in the next decade?
Yes, driven by consumer preferences for convenience, aging populations, and growing gastrointestinal health awareness. -
What strategies should companies adopt to profit from price variations globally?
Tailor product features to regional needs, establish strategic partnerships, leverage economies of scale, and focus on demonstrating clinical and economic value to justify premium pricing.
Sources
[1] Grand View Research, "Enema Market Size & Trends," 2022.
More… ↓
