Drug Price Trends for FEXOFENADINE-PSE ER
✉ Email this page to a colleague
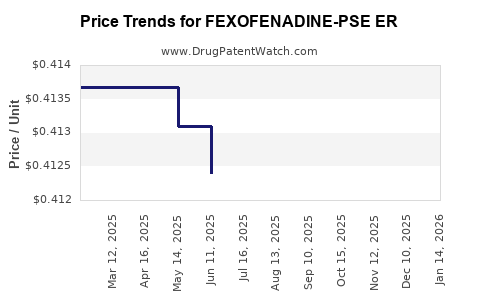
Average Pharmacy Cost for FEXOFENADINE-PSE ER
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| FEXOFENADINE-PSE ER 60-120 TAB | 43598-0823-14 | 0.43014 | EACH | 2024-11-20 |
| FEXOFENADINE-PSE ER 180-240 TB | 00536-1394-26 | 0.86690 | EACH | 2024-11-20 |
| FEXOFENADINE-PSE ER 180-240 TB | 43598-0892-35 | 0.86690 | EACH | 2024-11-20 |
| FEXOFENADINE-PSE ER 60-120 TAB | 55111-0447-14 | 0.43014 | EACH | 2024-11-20 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |


