Share This Page
Drug Price Trends for EYE WASH SOLUTION
✉ Email this page to a colleague
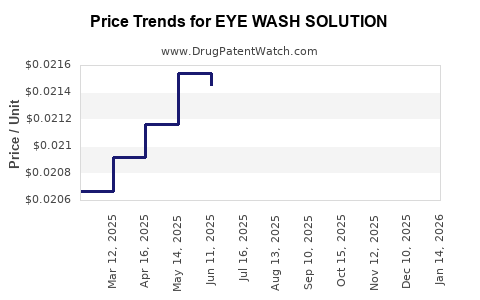
Average Pharmacy Cost for EYE WASH SOLUTION
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| EYE WASH SOLUTION | 00536-1224-97 | 0.02197 | ML | 2025-12-17 |
| EYE WASH SOLUTION | 00536-1224-97 | 0.02215 | ML | 2025-11-19 |
| EYE WASH SOLUTION | 00536-1224-97 | 0.02231 | ML | 2025-10-22 |
| EYE WASH SOLUTION | 00536-1224-97 | 0.02191 | ML | 2025-09-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
rket Analysis and Price Projections for Eye Wash Solution
Introduction
Eye wash solutions constitute a vital segment within the ophthalmic and first aid product markets, addressing immediate ocular exposure to chemicals, irritants, or foreign particles. Their importance spans industrial, healthcare, and consumer sectors. As the demand for safety and eye care products escalates globally, a comprehensive analysis of the market landscape and pricing dynamics becomes crucial for stakeholders seeking to navigate growth opportunities.
Market Overview
The eye wash solution market is characterized by steady growth driven by increasing awareness regarding ocular safety, the expansion of industrial activities, and rising adoption of first aid products in households and workplaces. According to industry reports, the global eye wash market was valued at approximately USD 300 million in 2022 and is projected to reach around USD 440 million by 2030, exhibiting a CAGR of roughly 4.5% during the forecast period [1].
Segmentation and Key Players
The market segments include consumer (retail), medical (hospital), and industrial sectors. Leading manufacturers like Johnson & Johnson, HIS Beauty & Medical Ltd., and Himalaya Wellness Co. dominate the landscape, with numerous regional and private-label brands contributing to product availability and affordability.
Regional Dynamics
North America and Europe hold significant market shares owing to stringent safety regulations, high awareness, and product proliferation. Asia-Pacific, however, demonstrates the fastest growth rate driven by expanding industrial operations, urbanization, and increased healthcare investments. Emerging markets such as India and China are witnessing heightened demand for affordable eye care solutions, further fueling regional market expansion [2].
Market Drivers and Challenges
Key Drivers:
- Rising Workplace Safety Regulations: Industrial sectors emphasize eye protection, leading to increased procurement of eye wash stations and portable solutions.
- Consumer Awareness: Growing knowledge about eye safety and first aid protocols in households and educational institutions.
- Regulatory Support & Standards: Governments and safety agencies setting standards for eye safety promote consistent product adoption and growth.
Challenges:
- Product Standardization: Variability in formulation and quality across regions and brands poses challenges in consumer trust and regulatory compliance.
- Pricing Pressure: Entry of low-cost generic products impacts profit margins and market share for premium offerings.
- Shelf Life & Preservation: Maintaining efficacy without preservatives, especially in multi-use solutions, presents formulation challenges.
Pricing Trends and Projections
Current Price Landscape
The retail price of eye wash solutions varies significantly based on factors like volume, formulation (sterile vs. non-sterile), packaging, and brand positioning. Typical price ranges are:
- Consumer-Grade (Retail): USD 3-8 for 4-8 oz bottles.
- Medical-Grade (Hospital/Clinic): USD 10-25 per unit, often higher for sterile, preservative-free formulations.
- Industrial/Commercial: Custom pricing, often included within first aid stations or bulk procurement.
Price Drivers
- Formulation & Sterility: Sterile, preservative-free solutions command higher prices due to manufacturing complexities.
- Packaging & Convenience: Portable, single-use vials or spray bottles increase costs relative to bulk solutions.
- Regulatory Compliance: Certification and quality standards, especially in medical applications, influence final pricing.
Future Price Projections
Analysts forecast a steady decline in unit costs driven by manufacturing scale-up and intensified competition, while premium, specialized products are expected to retain or elevate their pricing points. By 2030, the average retail price of standard eye wash bottles may dip by approximately 10-15%, reaching USD 2.80-7.00 per bottle in mass retail sectors due to commoditization [3]. Conversely, sterile, preservative-free solutions for medical use could sustain or surpass current prices due to regulatory hurdles and manufacturing costs.
Emerging trends such as pre-packaged, single-use eye washes with antimicrobial properties could command premium pricing, potentially reaching USD 12-15 per unit in specialty channels.
Market Opportunities and Strategic Implications
- Innovation in Formulation: Developing preservative-free, multi-use, sterile solutions aligned with regulatory standards can position manufacturers favorably in higher-price segments.
- Regional Expansion: Addressing unmet needs in Asian and Latin American markets offers growth potential, especially through affordable, locally-sourced solutions.
- Brand Differentiation: Emphasizing safety certifications, eco-friendly packaging, and ergonomic design can justify premium pricing and build consumer loyalty.
Regulatory Considerations
Compliance with standards such as ANSI/ISEA Z358.1 (for eyewash stations) and FDA regulations in the U.S., alongside ISO certifications, remains critical. International markets may require adherence to local safety mandates, impacting production costs and pricing strategies.
Key Takeaways
- The eye wash solution market is projected to grow at a CAGR of approximately 4.5% through 2030, driven by industrial safety, consumer awareness, and expanding healthcare infrastructures.
- Price points vary widely, with consumer-grade solutions costing USD 3-8 per bottle and medical-grade, sterile options costing substantially more.
- Future pricing trends suggest a marginal decline in standard solutions due to increased competition, with premium, sterile products maintaining higher price levels.
- Innovation, regional expansion, and regulatory compliance constitute key opportunities for market players to enhance margins and capture growth.
- Cost-effective, safety-compliant products tailored to regional needs will be critical to gaining competitive advantage in emerging markets.
FAQs
Q1: What are the primary factors influencing the pricing of eye wash solutions?
A: Formulation sterility, packaging convenience, brand reputation, regulatory compliance, and manufacturing costs are key determinants of retail and wholesale pricing.
Q2: How is the current global demand for eye wash solutions expected to evolve?
A: Demand is expected to grow steadily, fueled by increasing industrial safety standards, consumer awareness campaigns, and expanding healthcare infrastructure, especially in emerging markets.
Q3: Which regions are promising for market expansion for eye wash manufacturers?
A: Asia-Pacific, Latin America, and parts of Africa present strong growth opportunities due to rising industrial activities, urbanization, and healthcare investments.
Q4: What emerging innovations could impact future pricing dynamics?
A: Development of preservative-free, multi-use formulations with antimicrobial properties and eco-friendly packaging could allow manufacturers to command premium prices.
Q5: What regulatory standards should manufacturers be aware of in the eye wash market?
A: Standards such as ANSI/ISEA Z358.1, FDA regulations in the U.S., ISO standards for medical devices, and local safety regulations in target markets are critical considerations.
Sources
[1] Global Market Insights, “Eye Wash Market Size & Share Analysis,” 2022.
[2] Research and Markets, “Ophthalmic and Eye Care Products Market Outlook,” 2021.
[3] Bloomfield Securities, “Pricing Strategies in Medical Devices,” 2020.
More… ↓
