Share This Page
Drug Price Trends for EYE DROPS
✉ Email this page to a colleague
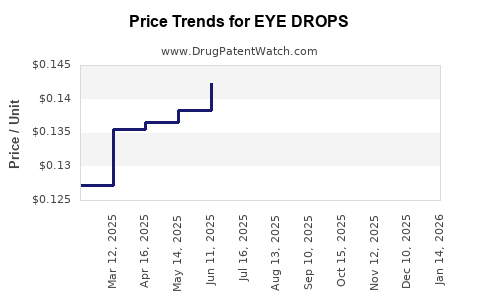
Average Pharmacy Cost for EYE DROPS
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| EYE DROPS | 24385-0077-05 | 0.14557 | ML | 2025-12-17 |
| EYE DROPS 0.05% | 70000-0454-01 | 0.09689 | ML | 2025-12-17 |
| EYE DROPS ADVANCED RELIEF | 70000-0456-01 | 0.14557 | ML | 2025-12-17 |
| EYE DROPS 0.05% | 00536-1217-94 | 0.09689 | ML | 2025-12-17 |
| EYE DROPS | 24385-0075-05 | 0.09689 | ML | 2025-12-17 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for Eye Drops
Introduction
The global eye drops market has experienced substantial growth driven by increasing prevalence of ocular conditions, expanding aging populations, and technological advancements in ophthalmic pharmaceuticals. Eye drops are a primary modality for managing conditions such as glaucoma, dry eye syndrome, allergic conjunctivitis, and post-surgical ocular care. This report assesses current market dynamics and projects future pricing trajectories for eye drops, providing strategic insights for pharmaceutical companies, investors, and stakeholders.
Market Overview
Global Market Size and Growth Trends
The global eye drops market was valued at approximately USD 17 billion in 2022 and is projected to reach USD 23 billion by 2030, expanding at a compound annual growth rate (CAGR) of over 4.0%[1]. The primary growth drivers include rising incidence of eye diseases, increased awareness, and technological innovations such as preservative-free formulations and sustained-release systems. North America currently dominates the market due to high healthcare expenditure and advanced ophthalmic infrastructure, followed by Europe and Asia-Pacific regions.
Key Drivers
- Prevalence of Ocular Diseases: Age-related macular degeneration, diabetic retinopathy, glaucoma, and dry eye syndrome are surging globally, necessitating ongoing treatment with eye drops.
- Aging Population: Older adults are more susceptible to ocular conditions, bolstering demand for eye drops[2].
- Innovations: The development of targeted, preservative-free, and sustained-release formulations enhances patient adherence and expands market scope.
- Healthcare Accessibility: Improved access to healthcare services in emerging economies fuels demand.
Market Segments
- Product Type: Prescription (Rx) eye drops dominate market share, including anti-glaucoma, anti-allergic, and antibiotic formulations. Over-the-counter (OTC) options are also expanding.
- Application: The largest segments are glaucoma management and dry eye syndrome. Other applications include allergy relief and post-surgical care.
- Distribution Channel: Pharmacies, hospitals, online platforms, and clinics.
Regulatory and Competitive Landscape
Regulatory Environment
Regulatory frameworks influence drug approvals, pricing, and reimbursement policies. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) impose rigorous testing requirements, impacting time-to-market and pricing strategies. The introduction of biosimilars and generic formulations exerts downward pressure on prices.
Competitive Landscape
Major players include Allergan (AbbVie), Bausch + Lomb, Santen Pharmaceutical, and Alcon. These companies differentiate through patent protections, R&D investments, and strategic partnerships. Generic entrants have increased price competition, especially in mature markets.
Pricing Dynamics of Eye Drops
Factors Influencing Price
- Formulation Complexity: Specialty formulations such as preservative-free or sustained-release drops cost more to produce, translating to higher prices.
- Regulatory Status: Patented drugs carry premium pricing due to exclusivity; generics are priced more competitively.
- Market Maturity: Emerging markets often see lower prices driven by regulatory and economic factors.
- Reimbursement Policies: Insurance coverage influences retail prices and patient affordability.
Current Price Range
- Brand-Name Prescriptions: Typical eye drops like those for glaucoma or dry eye retail between USD 60 and USD 150 per bottle.
- Generic Formulations: Can range from USD 10 to USD 40 per bottle, depending on branding and formulation complexity.
- Over-the-Counter Options: Usually priced between USD 10 and USD 30.
Price Trends and Drivers
- Patent Expirations: The expiration of blockbuster drugs will likely reduce prices via generics entering the market.
- Manufacturing Advances: Cost reductions through streamlined production could enable more competitive pricing.
- Market Competition: Increased competition drives downward pressure, particularly in OTC segments.
- Premiumization: Innovative offerings with superior efficacy or convenience command premium pricing.
Future Price Projections
Regional Variations
- North America and Europe: Prices are expected to stabilize with modest declines due to generics, but premium formulations may sustain higher margins.
- Asia-Pacific: Rapid growth in healthcare infrastructure and local manufacturing could lead to more competitive pricing and increased consumption, particularly in developing markets.
Projection Highlights (2023-2030)
- Prescription Eye Drops: Prices are projected to decline by 15-20% in mature markets due to generic entry but may maintain premium prices for innovative products.
- Over-the-Counter Drops: Prices could decrease by 10-15% as market saturation increases and manufacturing efficiencies improve.
- Premium Formulations: Continued investment in advanced delivery systems may sustain higher prices, with some niche products maintaining premiums of 20-30% over standard options.
Strategic Considerations
- Companies should invest in R&D to develop differentiated, patent-protected formulations to command premium prices.
- Monitoring regulatory changes is critical to anticipate pricing adjustments and reimbursement strategies.
- Market entry into emerging regions could unlock high-growth opportunities at relatively lower price points.
- The increasing prevalence of generics emphasizes need for cost-effective manufacturing and supply chain optimization.
Conclusion
The eye drops market is poised for steady growth, driven by demographic shifts and technological progress. While pricing pressures exist due to generics and competitive dynamics, innovation and strategic regional expansion afford potential for sustained revenue streams. Stakeholders should focus on differentiating offerings and navigating regulatory landscapes to optimize pricing strategies over the coming years.
Key Takeaways
- The global eye drops market is forecasted to grow at a CAGR exceeding 4% through 2030, reaching circa USD 23 billion.
- Prescription drug prices currently range from USD 60–150, with generics and OTC options being more affordable.
- Price trends indicate modest declines due to patent expirations, competition, and manufacturing efficiencies, particularly in mature markets.
- Innovative formulations and regional expansion into emerging markets present opportunities for premium pricing and increased profitability.
- Evolving regulatory policies and reimbursement frameworks are pivotal factors influencing future price dynamics.
FAQs
1. What are the primary factors influencing the pricing of eye drops?
Formulation complexity, regulatory status, market maturity, competition, and reimbursement policies are pivotal.
2. How will patent expirations affect eye drop prices?
Patent expirations typically lead to increased generic competition, exerting downward pressure on prices.
3. Which regions are expected to see the fastest growth in eye drop consumption?
Asia-Pacific and other emerging markets are projected to experience rapid growth due to expanding healthcare infrastructure.
4. What innovations could justify premium pricing for eye drops?
Preservative-free formulations, sustained-release delivery systems, and targeted therapies can command higher prices.
5. How do regulatory changes impact the market and pricing?
Stringent approval processes may delay product launches and influence pricing strategies; conversely, favorable reforms can expedite market entry and competitiveness.
Sources:
- Market Research Future, "Eye Care Market Forecast," 2022.
- WHO, "Vision Health Data," 2021.
More… ↓
